This article has been corrected. See "CORRIGENDUM: Correction of 4th author's name: The Krüppel-like factor (KLF5) as a predictive biomarker in preoperative chemoradiation therapy for rectal cancer" in Volume 97 on page 157.
Abstract
Purpose
Preoperative chemoradiation therapy (CRT) has become the standard treatment for patients with locally advanced rectal cancer, 15%–30% of patients still progress while being treated with CRT. The aim of this study was to identify as important biomarker of poor response and evaluate the mechanism associated with CRT resistance.
Methods
This study included 60 human colon tumour pre-irradiation specimens. Expressions of epidermal growth factor receptor (EGFR), p53, Krüppel-like factor 5 (KLF5), C-ern, Ki67 were assessed and correlated with tumor regression grades and complete remission. We added in vitro study with biomarker which has been identified as important biomarker of poor response to evaluate the mechanism associated with CRT resistance.
Results
Pathologic complete remission (pCR) was achieved by 9 patients (18%). EGFR and KLF5 were significantly associated with pCR (P = 0.048, P = 0.023, respectfully). And multivariate analysis showed high KLF5 intensity was worse factor for pCR (P = 0.012). In vitro study, radiation or chemotherapy therapy stabilized KLF5 protein levels in a time- and dose-depended manner in HCT116 and Caco-2 cells. KLF5 overexpression in HCT116 stable cell line showed significantly better cell viability by increasing cyclinD1 and b-catenin compared to control cells in MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, suggesting that KLF5 mediates cell survival.
Preoperative chemoradiation therapy (CRT) has become the standard treatment for patients with locally advanced rectal cancer [12]. This treatment has been shown to improve survival and may reduce local recurrence rates. Many studies have suggested that a patient's tumor regression grade is significantly associated with prognosis; specifically, patients with complete regression have a good prognosis [34]. However, 15%–30% of patients still progress while being treated with CRT or go on to develop distant metastasis [5678]. Therefore, predicting tumor response before treatment can significantly influence the selection of patients for preoperative CRT as well as potentially modify postoperative treatment plans. However, the currently available imaging modalities, including endorectal ultrasound, CT, MRI, or positron emission tomography used to restage patients after preoperative CRT lack the accuracy needed for this prediction [910]. For this reason, oncologists have a great interest in identifying molecular predictors of rectal cancer response to CRT [1112]. Many molecular markers have been investigated, but as yet, the availability of markers able to predict CRT response is lacking.
Recently Hur et al. [13] reported that incorporating multiple significant prognostic factors could increase the accuracy of predicting tumor response to CRT. In other words, one factor alone cannot explain or predict tumor response to CRT, suggesting that determining the detailed mechanism of tumor response to CRT is necessary.
The aim of this study was to determine useful predictive marker of tumor response to patients using biopsy immunohistochemistry (IHC) before irradiation. Furthermore, we added in-vitro study with biomarker which has been identified as important biomarker of poor response to evaluate the mechanism associated with CRT resistance.
From March 2015 to January 2016, four multicenter 60 consecutive patients who received neoadjuvant chemoradiation for locally advanced (radiological T3–4 or N+ and/or clinically bulky) rectal cancer were enrolled in this study. Patients with distant metastases, recurrent disease, previous chemotherapy, pelvic radiotherapy, abnormal liver, kidney, or bone marrow function, or those aged less than 18 years or more than 80 years, were excluded. The study was approved by the scientific review and ethics committee at our institution. Written informed consent was obtained from all the patients before the study. The initial clinical stage was based on a digital rectal examination, rigid proctoscopy, abdominopelvic CT, pelvic magnetic resonance imaging, chest CT, whole-body positron emission tomography/CT, complete blood cell count, liver function tests, and the serum CEA level. The location of the tumor was defined as the distance between the caudal margin of the tumor and the anal verge, and this was measured by a digital examination and rigid proctoscopy. A biopsy was performed before starting CRT. Approval for all research-related activities was obtained from the Institutional Review Board of the Hallym University (approval number: 2019-05-002).
All patients received a total dose of 50.4 Gy with daily fractions of 180 cGy/d over 5 weeks. Chemotherapy was administered intravenously, consisting of 5-fluorouracil (5-FU; 425 mg/m2/day) and leucovorin (20 mg/m2/day) during the first and fifth weeks of radiotherapy. Experienced surgeons performed the radical surgery, which included total mesorectal excision, high vascular ligation (the inferior mesenteric artery and vein), and en bloc resection of the adjacent involved organs, 6 to 8 weeks following the completion of preoperative chemoradiation.
The surgery included low anterior resection with colorectal or coloanal anastomosis and abdominoperineal resection. Use of a diverting stoma was subject to the surgeon's decision. The disease staging was based on the final pathological features of the tumor according to the seventh Union for International Cancer Control TNM staging system. Two experienced gastrointestinal pathologists read the results of the pathology collected from the 4 institutions according to the pathologic TNM staging and the tumor regression grading (TRG) systems based on the ratio of fibrosis to residual cancer [14]. TRG scores were defined as follows: TRG1 (fibrosis <25%), TRG2 (25%≤fibrosis <50%), TRG3 (50%≤ fibrosis), and TRG4 (complete remission).
With pilot study, we selected biomarkers that differed more than 4 fold in mRNA levels in TRG4 and TRG1. Epidermal growth factor receptor (EGFR), C-ern and Ki67 and Krüppel-like factor 5 (KLF5), known as a transcriptional factor acting down-stream of RAS-MAPK cascade, were selected [15161718]. Biopsy slides were blocked with 5% bovine serum albumin in phosphate buffered saline (PBS) and primary antibodies (Abcam, Ltd., Cambridge, United Kingdom) were applied at a 1:50 dilution, followed by incubation with biotinylated anti-rabbit secondary antibody (Dianova, Hamburg, Germany, at a dilution of 1:50). With EGFR, C-ern, the intensity immunostaining was scored as: 1+ (weak), 2+ (moderate), and 3+ (intense). With Ki67 and KLF5, the percentage of positive tumor cells and staining intensity were then multiplied to produce a weighted score for each case ranging from 0 to 12; “low expression” was classified as a score of 5 or below and “high expression” as a score of 6 or above.
The human colorectal adenocarcinoma cell lines, SW48, HCT116 p53+/+, HCT116 p53−/−, Caco-2, DLD-1, and HT-29 were obtained from the American Type Culture Collection (LGC-Promochem, Wiesbaden, Germany). SNU-C4 5-FU-sensitive cell line and SNU-C4 5-FU-resistant cell line (which was generated by exposing cells to 5-FU for more than 6 months to create stable cell lines resistant to 5-FU), were obtained from Korean cell line bank (Seoul, Korea). The cells were maintained in Dulbecco's modified eagle medium (Biochrom, Berlin, Germany) and supplemented with 10% heat-inactivated fetal calf serum, 1% sodium pyruvate, and 2 mmol/L glutamine (all supplements from Biochrom) at 37℃, 5% CO2, and 95% humidity.
For immunoblotting, cells were washed and lysed in radioimmunoprecipitation assay buffer (50 mmol/L Tris [pH, 7.4], 150 mmol/L NaCl, 1% Triton X-100, 1% deoxycholate) supplemented with protease inhibitors (1-mmol /L phenylmethylsulfonylfluoride, 10-ag/mL pepstatin, 10-ag/mL aprotinin, and 5-ag/mL leupeptin; all from Sigma, Deisenhofen, Germany). Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Equal amounts of protein (10 ag) were separated on a 12.5% sodium dodecyl sulfate polyacrylamide gel and transferred to a nitrocellulose membrane (Hybond C, Amersham, Freiburg, Germany). Membranes were blocked in 5% nonfat dry milk in PBS for 30 minutes at room temperature and probed with rabbit anti-KLF5, KRAS, cyclin D1, b-catenin, a-tubulin, Trp53, P21, Bax, or PUMA antibodies (dilution, 1:1,000, R&D Systems, Wiesbaden, Germany) overnight at 4℃. Next, membranes were incubated with horseradish peroxidase-linked secondary antibodies (1:200, Dako, Hamburg, Germany) and developed by an enhanced chemoluminescence detection system (ECL, Amersham) and autoradiography (Biomax film, Kodak, Rochester, MN). To confirm equal protein loading, membranes were subsequently reprobed with a 1:2,000 dilution of an anti-h-tubulin antibody (Biozol, Eching, Germany) or GAPDH (Biozol, Eching, Germany). For densitometric analysis, scanned autoradiographs were quantified using the AIDA software package (Raytest, Straubenstadt, Germany).
For stable overexpression of the biomarker gene, the fragment encoding the full-length cDNA from the pSG5-vector construct was cloned into the SmaI-XhoI sites of pLL-CMV-puro lentiviral vector. Plasmid DNAs were transfected into HCT116 cells along with lentiviral packaging mix consisting of an envelope and packaging vector to produce lentivirus packed with biomarker cDNA.
Suspension cells were harvested by centrifugation for 5 minutes at 6℃. The cell was washed by resuspending in 5 mL sterile PBS. Then the cells were diluted from 5 × 106 to 5 × 103 cells/mL. The cells were incubated for 12 hours. And then 10 L of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent were added to each well. When purple precipitate is clearly visible, waiting 2 more hours, we measured the absorbance of the wells, including the blanks, at 570 nm.
Statistical evaluation was carried out using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Tumor regression and N down grading were tested with the chi-square test. The pathologic complete remission study was analyzed with binary logistic regression method. Comparison of factors to KLF5 scoring was used with t-test. A value of P < 0.05 was considered statistically significant.
The patient's demographics were tabulated (Table 1). A total of 60 patients were included in this study, 49 males and 11 females. The median age of the patients was 59 ± 18.3 years (range, 35–79 years), and the median distance of the tumor from the anal verge was 7.3 ± 3.1 cm. Thirty-four patients (56.7%) exhibited a poor response to CRT (TRG 1 or 2), whereas 26 patients (43.3%) exhibited a good response (TRG 3 or 4). A pathologic complete response (pCR) was observed in 9 patients (15%).
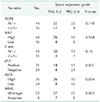
Tumors of 8 of the patients (13.3%) harbored a KRAS mutation. The p53 expression observed 31 patients (51,6%), High KLF5 expression observed 36 patients (60%). High KLF5 expression and p53 mutation were significantly associated with poor response of CRT (TRG 1 or 2) (P = 0.004, P = 0.021, respectfully) (Table 2). Additionally, a trend towards an association between KRAS mutation status and tumor regression was observed but was not statistically significant (P = 0.062).
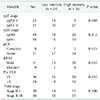
Clinical factors, as well as the protein expression of several tumor-related markers, were evaluated to determine association with pCR (Table 3). By univariate analysis, high EGFR and KLF5 expression were found to significantly correlate with failure of pCR (P = 0.048, P = 0.023); however, after multivariate analysis, only KLF5 expression still had significance as a poor prognostic factor.
Because KLF5 was determined to correlate with tumor response to treatment, we compared KLF5 expression to several clinical characteristics known to effect tumor response, including ypT and ypN stages, KRAS mutation status, pre-CEA, and TNM stage (Table 4). The high intensity of IHC staining of KLF5 significantly correlated with increasing ypT staging (P = 0.009) and high related tendency with KRAS mutation status (P = 0.055).
We examined 6 different human colorectal cancer cell lines for mutated KRAS, mutated BRAF and KLF5 expression. Through DNA sequencing, we determined that DLD-1, SW-48, HCT116 p53+/+, and HCT116 p53−/− contained KRAS activating mutations, while HT-29 and CaCo-2 contained wild-type KRAS. Only HT-29 contained a BRAF mutation. The level of KLF5 expression in the 6 cell lines correlated with KRAS genotype, with those containing mutated KRAS having higher levels. It is of interest to note that HT-29, which contains a mutated BRAF gene, exhibited higher levels of KLF5, similar to those cell lines harboring KRAS mutations. KRAS wild-type cell lines, SW48 and CaC02, had low levels of cyclin D1 and b-catenin, as well as low levels of KLF5. Conversely, HCT116 p53+/+ and HCT116 p53−/−, cell lines with activating KRASG13D mutations, exhibited higher levels of KLF5, cyclin D1 and b-catenin (Fig. 1A).
We evaluated the (cyclin D1, b-catenin) protein known as expressed at the downstream of KLF5 expression. The cell line with KRAS mutation such as HCT116+/+ or HCT116−/−, high KLF5 expression, high cyclin D1 and high b-catenin expression was shown sequentially. However, the cell line without KRAS mutation such SW480, CaC02 showed low KLF5 expression and cyclin D1 and b-catenin expressed in proportion to KLF5 expression (Fig. 1B).
To determine the effect of chemotherapy and radiation therapy on KLF, we selected Caco-2 (without KRAS mutation) and HCT116 cell line (with KRAS mutation) which have endogenous KLF5 protein. We compared KLF protein expression in HCT 116 (KRASG13D) cells treated with radiation (0, 2, 5, 10, 20 Gy). Additionally, we compared KLF expression in Caco-2 cell (wild-type KRAS) treated with or without 5-FU (Fig. 2B). We found that KLF5 protein levels increased soon after DNA damage: levels were maximal after 10 Gy of radiation in HCT116. Similarly, in HCT116 cell, KLF5 protein levels were maximal 24 hours after 10 nM 5-FU treatment in Caco-2 (Fig. 2A).
Additionally, we evaluated the changes of KLF5 protein expression levels between SNU-C4 and SNU-C4 5-FU. SNU-C4 5-FU is a stable cell line that has been subjected to long-term exposure of 5-FU and as a result, has developed 5-FU resistance (Fig. 2A). The level of KLF5 was increased in SNU-C4 5-FU compared to SNU-C4. Similarly, cyclin D1 and b-catenin were also increased (Fig. 2B).
We made a stable cell line with HCT116 which are overexpressed of KLF5 (HCT116 KLF5 OE). We performed an experiment similar to HCT116 p53+/+ cells, expressing endogenous KLF in HCT116 KLF5 OE). The Cells (HCT116 and HCT116 KLF5 OE) were subjected to 10-cGy radiation exposure, after which cell death protein levels were evaluated. The p53 was checked for radiation effect. After radiation therapy, cell death protein such as BAX, p53, PUMA tends to be increased compared to control in both groups. In both cell lines, cyclin D1, b-catenin increased proportional to the protein amount of KLF5 after radiation. Expected, cyclin D1 and b-catenin were increased after radiation, especially this increase was amplified in HCT116 KLF5 OE which would be proportional to a mount of baseline KLF5 protein (Fig. 3A).
Lastly, we did MTT assay to assess for cell survival. With MTT assay, HCT116 KLF5 OE cells exhibited a significantly higher survival rate compared to control HCT116 cells (P < 0.05), further suggesting that KLF5 mediates cell survival (Fig. 3B).
This study was to investigate the biomarker, predictive of response of preoperative chemotherapy in rectal cancer using the IHC stain, which is the easiest and quickest method. In this study, we have found that a novel factor, KLF5 as a poor prognosis factor for T-down staging, but it is an unfamiliar biomarker. So, we did experimental in vitro study why KLF5 expression is related with resistance to chemo or radiation therapy. In vitro, we found that the KLF5 is located downstream of the KRAS mutation and protein KLF5 is stabilized by chemo or radiation therapy, increasing the cell cycling protein such as cyclin D1 or cell proliferation protein such as b-catenin, which is predicted to be related to cell survival.
In recent years, several promising candidate markers have been reported potential roles in the prediction of radiation response, including angiogenesis (thymidine phosphorylase, thymidylate synthase), and vascular endothelial growth factor, proliferation (cyclooxygenase-2 and proliferating cell nuclear antigen) and cell adhesion or collagenase (CD44, CD133, matrix metalloproteinase [MMP] 2, and MMP9) and apoptosis (bax, p53, nuclear factor-kappa B [NfkB], and surviving) with regard to preoperative CRT in rectal cancer [1920]. Also, few studies have evaluated KRAS as a biomarker for tumor response in rectal cancer patients treated with CRT and TME. Garcia-Aguilar et al. [21] described a series of rectal cancer patients treated with preoperative CRT and reported that tumors with wild-type KRAS were more likely to respond to CRT than tumors with mutant KRAS.
KRAS mutation was reported 35%–40% in previous studies [2223] although we just observed only just 15%. As PETACC-8 already demonstrated, KRAS mutation is most important factor in treatment in colorectal cancer [24]. But, most of study showed KRAS mutation has no significant as a prognostic factor in preoperative CRT. As our data demonstrated, KRAS mutation tended to predict tumor regression grade but not has significance. Instead, KLF5 expression in IHC straining predict CRT response efficiently not only tumor regression, but also pathologic complete remission. The KLF5 is a zinc finger-containing transcription factor that is involved in diverse physiological processes including proliferation and differentiation of intestinal epithelial cells [16]. KLF5 is known to be increased by oncogenic KRASV12 and the BRAF-ERK-MEK cascade [18]. KLF5 has a pro-proliferative effect in cultured cells through activation of cell cycle regulatory proteins such as cyclin D1, cyclin B1, and Cdc2 [161718]. Here, we made a hypothesis that each mutation of RAS, RAF, MER, ERK also influence the results of chemoradiation response, so, molecule of downstream such like KLF5 would be more specific to predict CRT response. KLF5 is known to be a crucial role in the maintenance of cellular proliferation, cyto-differentiation, and morphology of the crypt-villus axis [2526]. And it is amplified in colorectal cancers suggesting a contributory role in tumorigenesis to regulate cell cycle components such as Cyclin D1, Cyclin B1, and Cdc2 [1625].
Our in vitro, we want to validate the independent role of KLF5 with stress. Previous report showed the increase of KLF 5 protein stability depends on dose of chemotherapy [27]. Same as previous report, protein level of KLF5 is increased as dose dependent with radiation therapy was inducted, in HCT116 which has KRAS mutation. We select Caco-2 cell line, which has wild type KRAS but just has a few levels of KLF5 protein. Interestingly, Caco-2 showed same tendency of increasing protein of KLF5 as time or dose dependent with treated 5-FU chemotherapy. We had a conclusion; a protein of KLF5 is directly influenced by stress. Here, important data was that the baseline protein level of KLF5 has decisive effect when stress (radiation, chemotherapy) would be added. Long exposure of 5-FU in SNU-C4 getting chemo-resistance showed high protein level of KLF5, sequentially increasing cyclin D1, b-catenin. We thought that KLF5 expression would trigger cell cycle activation via cyclin D1 or cyclin B1/CdC [16] and maintain bonding each other or adhesion another site via b-catenin. We preliminary suggest the positive role of KLF5 for to survive from CRT via increasing cyclin D1 related with cell cycle, b-catenin related with cell proliferation.
We made stable cell line with HCT116 which gets overexpress of KLF5. We made a stress with 10-cGy radiation in both HCT116 and HCT116 KLF5 OE. After 24 hours, HCT116 KLF OE showed apoptosis markers such as bax, P53, PUMA. The cyclin D1 or b-catenin amplified by chemotherapy for survival especially in HCT116 KLF OE. In MTT assay, HCT116 KLF5 OE stable cell line showed significantly better cell viability compared to control cells, suggesting that KLF5 mediates cell survival. We just analogize KLF5 has a potential role to survive following threatening signal, via promoting cell cycle through cycle D1, B1/CdC.
A recent systemic review emphasized neoadjuvant CRT should be considered in patients with high-risk locally advanced rectal cancer to reduce local recurrence and distant failure [28]. However, as mentioned above, 15%–30% of patients still progress while being treated with CRT or go on to develop distant metastasis, require different treatment regimen. Therefore, patients with poor response should be well selected, and required personalized treatment. Recently the important biomarker such as BRAF, SMAD4 gene mutation and polo-like kinase 1 expression [2930] are discussed to predict poor response to CRT and to clarify the mechanism of chemoradio-resistance. This mechanism studies could help to develop new therapeutic target to increase the response rate of CRT, and this ongoing effort will open a new chapter in rectal cancer treatment.
Our study has several limitations. First pathologic complete remission rate is just 15%, lower results than other papers. We needed more than 8 times biopsy for IHC before radiation. So, small sized rectal cancer could not be enrolled in this study. Second, it is retrospective study with low sample size to make a conclusion. Third, the use of KLF5 as a predicative biomarker may be limited because it affects T-down staging but not N-down staging. However, since KLF5 is a poor prognostic factor for T-down staging, it does not matter even if it is not related with N-down staging. Lastly, the experimental studies for evaluating mechanism are insufficient to conclude that KLF5 protein was directly related with chemoradio-resistance, but the attempt to indirectly suggest the mechanism of chemoradiation resistance is thought to be meaningful.
To the best of our knowledge, our study is the first article to explain the mechanism how tumor can survive in threatened stress like CRT. We found that protein of KLF5 which related with KRAS, BRAF mutation is a potential mediator to resist CRT in rectal cancer. And that reason, KLF5 expression was could be a specific biomarker to predict poor TRG response after CRT in rectal cancer patients. We thought KLF5 protein has been amplified following CRT and disturb tumor down grading to make active cell cycle via cyclin D1, cyclin B1 and Cdc2 or b-catenin.
In conclusion, overexpression of KLF5 in pretreatment biopsies might be predictive factor of poor tumor regression after preoperative CRT. KLF5 was significantly related with KRAS mutation but, KLF5 has an independent role to get resistance from preoperative CRT. Our study suggested one possible mechanism of biomarker to predict chemoradiation response.
Figures and Tables
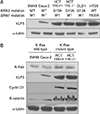 | Fig. 1KLF5 and up/down-stream on colon cancer cell line. (A) Western of KLF5 expression depends on KRAS and BRAF mutation. (B) The protein expressed at the downstream of KLF5 with KRAS or without KRAS mutation cell line. KLF, Krüppel-like factor; WT, wild type. |
 | Fig. 2The changes of KLF5 expression by stress induction in colon cancer cell line. (A) Radiation therapy, chemotherapy increased KLF5 depends on dose or time regardless KRAS mutation. (B) The changes in protein of KLF5 in SNU-C4 after getting chemoresistance. KLF, Krüppel-like factor; 5-FU, 5-fluorouracil; RT, radiation therapy. |
 | Fig. 3The survival analysis with induction of apoptosis signal. (A) Radiation therapy in HCT116 and HCT116 KLF5 OE. (B) HCT116 KLF5 OE shows survival benefits in Cell death signals in MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. KLF, Krüppel-like factor. *P < 0.05. |
Table 2
The biomarker expression in tumor tissue for assessment of tumor regression grade and down grading
References
1. Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997; 336:980–987.

2. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radio therapy in rectal cancer. N Engl J Med. 2006; 355:1114–1123.
3. Kaminsky-Forrett MC, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998; 42:935–941.

4. Yoon WH, Kim HJ, Kim CH, Joo JK, Kim YJ, Kim HR. Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer. Ann Surg Treat Res. 2015; 88:15–20.

5. Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002; 53:664–674.

6. Reerink O, Karrenbeld A, Plukker JT, Verschueren RC, Szabo BG, Sluiter WJ, et al. Molecular prognostic factors in locally irresectable rectal cancer treated preoperatively by chemo-radiotherapy. Anticancer Res. 2004; 24:1217–1221.
7. Crane CH, Skibber JM, Feig BW, Vauthey JN, Thames HD, Curley SA, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003; 97:517–524.

8. Wheeler JM, Dodds E, Warren BF, Cunningham C, George BD, Jones AC, et al. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: correlation with rectal cancer regression grade. Dis Colon Rectum. 2004; 47:2025–2031.

9. Huh JW, Park YA, Jung EJ, Lee KY, Sohn SK. Accuracy of endorectal ultrasonography and computed tomography for restaging rectal cancer after preoperative chemoradiation. J Am Coll Surg. 2008; 207:7–12.

10. Huh JW, Min JJ, Lee JH, Kim HR, Kim YJ. The predictive role of sequential FDG-PET/CT in response of locally advanced rectal cancer to neoadjuvant chemoradiation. Am J Clin Oncol. 2012; 35:340–344.

11. Negri FV, Campanini N, Camisa R, Pucci F, Bui S, Ceccon G, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer. 2008; 98:143–147.

12. Bertolini F, Bengala C, Losi L, Pagano M, Iachetta F, Dealis C, et al. Prognostic and predictive value of baseline and posttreatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007; 68:1455–1461.

13. Hur H, Kim NK, Min BS, Baik SH, Lee KY, Koom WS, et al. Can a biomarker-based scoring system predict pathologic complete response after preoperative chemoradiotherapy for rectal cancer? Dis Colon Rectum. 2014; 57:592–601.

14. Rödel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005; 23:8688–8696.

15. Bateman NW, Tan D, Pestell RG, Black JD, Black AR. Intestinal tumor progression is associated with altered function of KLF5. J Biol Chem. 2004; 279:12093–12101.

16. Nandan MO, Chanchevalap S, Dalton WB, Yang VW. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005; 579:4757–4762.
17. Nandan MO, Yoon HS, Zhao W, Ouko LA, Chanchevalap S, Yang VW. Krüppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004; 23:3404–3413.

18. Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, et al. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008; 134:120–130.
19. Qiu H, Sirivongs P, Rothenberger M, Rothenberger DA, Garcia-Aguilar J. Molecular prognostic factors in rectal cancer treated by radiation and surgery. Dis Colon Rectum. 2000; 43:451–459.

20. Lin JT, Chang TH, Chang CS, Wang WH, Su BW, Lee KD, et al. Prognostic value of pretreatment CD44 mRNA in peripheral blood of patients with locally advanced head and neck cancer. Oral Oncol. 2010; 46:e29–e33.

21. Garcia-Aguilar J, Chen Z, Smith DD, Li W, Madoff RD, Cataldo P, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg. 2011; 254:486–492.

22. Rosty C, Young JP, Walsh MD, Clendenning M, Walters RJ, Pearson S, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013; 26:825–834.

23. Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009; 6:519–527.

24. Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:862–873.

25. Liu Y, Wen JK, Dong LH, Zheng B, Han M. Kruppel-like factor (KLF) 5 mediates cyclin D1 expression and cell proliferation via interaction with c-Jun in Ang II-induced VSMCs. Acta Pharmacol Sin. 2010; 31:10–18.
26. Bell KN, Shroyer NF. Krupple-like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation. Dig Dis Sci. 2015; 60:86–100.
27. Zhao Y, Hamza MS, Leong HS, Lim CB, Pan YF, Cheung E, et al. Kruppel-like factor 5 modulates p53-independent apoptosis through Pim1 survival kinase in cancer cells. Oncogene. 2008; 27:1–8.

28. Tut TG, Lim SH, Dissanayake IU, Descallar J, Chua W, Ng W, et al. Upregulated polo-like kinase 1 expression correlates with inferior survival outcomes in rectal cancer. PLoS One. 2015; 10:e0129313.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download