Abstract
Purpose
Colon perfusion status is one of the most important factors for the determination of postoperative anastomotic complications. Colonic hypoperfusion can be induced by inferior mesenteric artery (IMA) ligation in some patients. This study aimed to evaluate atherosclerotic risk assessment and vascular parameters of CT angiography as predictors of colonic hypoperfusion.
Methods
This prospective study was conducted at a tertiary referral hospital and included 46 rectosigmoid colon cancer patients undergoing laparoscopic anterior resection between August 2013 to July 2014. Atherosclerotic risk scores were assessed using the Framingham cardiovascular risk score system. The IMA length, branching pattern, atherosclerotic calcification, and intermesenteric artery and mesenteric vascular diameters were evaluated using CT angiography. Mesenteric marginal artery pressures were measured before and after IMA clamping. The mean arterial pressure (MAP) index was calculated by dividing the mesenteric marginal MAP into the systemic MAP to determine the mesenteric hypoperfusion status after IMA clamping. A critically low MAP index was defined as <0.4.
Results
Critically low MAP index (<0.4) was observed in 6 cases (13.0%) after IMA clamping. Atherosclerotic calcification of the IMA and superior mesenteric artery occurred in 11 (23.9%) and 5 patients (10.9%), respectively. Low MAP index was associated with high atherosclerotic risk score and short IMA length, rather than atherosclerotic calcification and other vascular parameters of the major mesenteric arteries. Multivariate analysis indicated that high atherosclerotic risk and short IMA length were independent predictors of critically low MAP index.
Radical lymphadenectomy is expected to improve oncologic outcomes of patients with advanced rectosigmoid cancer [1]. High ligation of the inferior mesenteric artery (IMA) from the aorta is widely performed during laparoscopic rectosigmoid colon cancer surgery as it permits safe and easy execution of radical D3 lymphadenectomy [2].
Ligation of the IMA for low anterior resection may induce colonic hypoperfusion. Colon perfusion status is one of the most important factors for the determination of postoperative anastomotic complications, including leakage, stricture, or colon ischemia, which occurred in 10% of the patients [3]. To maintain favorable colon perfusion, low IMA ligation can be selected for preservation of the left colic artery (LCA) [45].
However, there are no standard mesenteric hemodynamic parameters that can be used to predict mesenteric hypoperfusion, which causes colon ischemia. Few studies on mesenteric hemodynamics have been reported. To date, the scope of clinical studies has been limited due to the difficulty in observing the colonic hypoperfusion status during surgery. Therefore, several different clinical studies have failed to reach a consensus for IMA ligation level and have reported contradictory results [567].
The ability to identify patients at risk of critical mesenteric hypoperfusion before IMA ligation would be very helpful for surgeons when determining the IMA ligation level. CT angiography can be used to evaluate vascular anatomical structures and arterial atherosclerotic changes that may be related to the disturbance of mesenteric perfusion [8]. The frequency of colonic ischemia was greater following aortic endovascular repair of aortic aneurysm than that following colorectal surgery [9]. Atherosclerotic cardiovascular disease and colonic ischemia have similar risk factors [10]. In aortic aneurysm patients, ischemic colitis was reported to occur when the IMA stump pressure was less than 40% of the systemic artery pressure. However, it is unclear whether arterial atherosclerotic change can predict critical mesenteric hypoperfusion in patients with rectosigmoid cancer.
Thus, we evaluated mesenteric artery properties on CT angiography and atherosclerotic risk assessment as predictors of hemodynamic change according to IMA ligation.
This prospective study involved 46 patients who underwent laparoscopic anterior resection between August 2013 and July 2014. Patients were included if they were aged 40–79 years and had stages I–III rectosigmoid colon cancer. The exclusion criteria were emergency surgery, open conversion, abdominal aortic aneurysm, severe cardiopulmonary disease, and pregnancy. All patients were followed-up for at least 3 years postoperatively. This study was conducted with the approval of the Institutional Review Board (approval number: 05-2013-045) of the Pusan National University Yangsan Hospital. Written informed consent was obtained from all patients.
Atherosclerotic risk assessment was performed using the Framingham 10-year general cardiovascular risk score system based on clinical characteristics of patients, including age, sex, smoking, hypertension, diabetes, systolic blood pressure (SBP), total cholesterol, and HDL [11]. The Framingham cardiovascular risk score system is widely used to predict the risk of cardiovascular disease. The revised risk score system improved the predictability of cardiovascular disease as well as cerebrovascular events, peripheral artery disease, and heart failure [11]. Therefore, this risk score system was applied as a clinical assessment tool for combined atherosclerotic risk factors. High atherosclerotic risk was defined as more than 20 points on the Framingham risk score system [12].
The CT scanners used were Somatom Definition AS+scanners (Siemens Medical Systems, Erlangen, Germany). Arterial and portal venous-phase dynamic CT images were obtained for all patients. For contrast enhancement, 100–120 mL of 300–370 mg/mL iopromide (Ultravist 300 or 370; Bayer Schering Pharma, Berlin, Germany) was administered intravenously at 3–4 mL/sec using an automatic power injector through an 18-gauge intravenous cubital line, followed by a 20-mL saline flush at the same flow rate. Imaging delays used for the arterial phase and portal venous phase were 15 and 25 seconds, respectively. These were counted after the descending aorta enhancement reached 100 Hounsfield units using a bolus-tracking method. The tube voltage was 120 kV and the maximum tube current was 400 mA, using an automated dose reduction system. Axial images and coronal multiplanar reconstruction images were reconstructed using a 3-mm slice thickness and maximum intensity projection views were created on a separate commercially available console system using 3-dimensional image.
The variations of IMA branches were assessed using the classification for IMA bifurcation patterns [13]. In type 1, the LCA first branched from the IMA trunk, regardless of the sigmoid artery (SA). In type 2, the LCA and SA branched from the common branch of the IMA trunk. In type 3, the LCA, SA, and superior rectal artery (SRA) branched at the same point in the common trunk of the IMA. In type 4, the LCA was absent (Fig. 1). The vascular patency of the LCA was evaluated using conventional enhanced CT imaging 1 year after surgery.
We identified the presence of the intermesenteric artery including Arc of Riolan or accessary middle colic artery (MCA) between the LCA and MCA or superior mesenteric artery (SMA). The IMA length was measured from the origin to the first branch. Internal diameters of the mesenteric vessels (IMA, SMA, LCA, MCA, and intermesenteric artery) were measured at a position within 1 cm of the branching point in an image magnified 500 times in the axial view mode (Fig. 1).
Atherosclerosis grades of the mesenteric arteries were scored according to the maximum calcification of the circumference in the axial cut surface of the artery [14]. Positive arterial calcification was defined as >25%. All radiologic evaluations were performed by 2 radiologists who had no prior knowledge of the clinical information.
Apical lymph node dissection around the IMA was performed for pathologic staging. Dissection was continued along the IMA to the first bifurcation point, and the branches of the IMA were identified. The SRA and SA were ligated while preserving the LCA (Fig. 2A). Following rectal transection, the rectosigmoid colon was extracted through the transumbilical mini-laparotomy site. After determining the proximal resection margin of the colon, the remaining colonic mesentery was divided to leave a marginal artery (Fig. 2B). A 24-gauge intravascular catheter (BD Angiocath Plus, BD Medical, Sandy, UT, USA) was cannulated into the mesenteric marginal artery and connected to a blood pressure (BP) transducer that was linked to the patient monitoring system (Intellivue MP80; Philips, Eindhoven, the Netherlands). The IMA was clamped using a laparoscopic atraumatic endo vessel clip (PL548S; Aesculap Inc., Tuttlingen, Germany) for high ligation. Hemodynamic changes of the mesenteric marginal artery were monitored before and after IMA clamping. SBP and diastolic BP (DBP) were measured in the mesenteric marginal artery according to IMA clamping, and the mean arterial pressure (MAP) was calculated using this following equation:
MAP = (SBP + 2 × DBP)/3.
Systemic MAP was also measured in the radial artery of the patient's wrist. Ischemic colitis was reported to occur when the mean IMA/systemic BP ratio was less than 0.4 in aortic aneurysm patients [15]. Anastomotic leakage was also reported with a MAP ratio less than 0.4 after left colon resection [16]. Thus, in this study, we adopted the MAP index as the measurable outcome of critical hemodynamic change. The MAP index was calculated by dividing the mesenteric marginal MAP into systemic MAP as follows: MAP index = marginal MAP/systemic MAP. A critically low MAP index was defined as a MAP index less than 0.4 after IMA clamping.
A paired t-test was used to compare MAP before and after IMA clamping. An unpaired t-test was used to compare MAP between patients. Chi-square and Pearson correlation tests were used for categorical variables. Regression analysis was used to evaluate the correlations of the atherosclerotic risk score and IMA length with hemodynamic alteration. Multivariate analysis was performed with a logistic regression model using a backward step-wise approach. The covariance input criterion was less than 0.1 and the elimination criterion was less than 0.05. Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA) and P < 0.05 was considered significant.
The mean age of colorectal cancer patients was 62.0 years, and the male-to-female ratio was 1.9:1. The Framingham risk score was 13.4 points on average, which was calculated by combining various clinical factors related to atherosclerotic risk. A total of 14 patients (30.4%) had high atherosclerotic risk defined as a score of 20 points or more. Anastomotic leakage occurred in 3 patients (6.5%); however, ischemic colitis was not found. The mean number of retrieved apical IMA lymph nodes was 4.0 ± 3.5 (0–18), and metastatic apical lymph nodes were found in 2 patients (Table 1). Analysis of previous medical history revealed that 13 patients were treated for hypertension (28.3%), and 3 patients experienced ischemic heart disease (6.5%) including acute myocardial infarction and angina pectoris. However, no patients had chronic kidney disease.
CT angiographic findings revealed that IMA type I was the most common branching pattern (Table 2). The mean IMA length was 35.5 mm, which was significantly associated with the IMA branching pattern (P = 0.041). The IMA length of the type III branching pattern (28.8 ± 10.9 mm) was significantly shorter than that of the IMA type I (36.4 ± 10.3 mm) and type II (40.9 ± 11.4 mm). The intermesenteric artery was identified in 11 patients (23.9%). LCA patency rates were 97.8% and 95.7% at 6 and 12 months, respectively. The internal diameter ratio between the IMA and SMA was 0.59 ± 0.14 (0.30–0.89) and the internal diameter ratio between the LCA and IMA was 0.62 ± 0.15 (0.25–0.96). Atherosclerotic IMA calcification occurred in 11 patients (23.9%), and 5 of them (10.9%) also had SMA calcification.
The mesenteric marginal MAP was steadily reduced by IMA clamping in all patients. In 6 cases (13.5%), the marginal MAP was less than 40 mmHg before IMA clamping; however, such marginal MAP values were observed in 15 cases (32.6%) after IMA clamping. There were significant differences in marginal MAPs before and after IMA clamping (56.5 ± 13.4 mmHg vs. 46.6 ± 13.7 mmHg, P < 0.001). The MAP indices were significantly different depending on the IMA clamping status (0.74 ± 0.15 vs. 0.61 ± 0.16 for before and after IMA clamping, P < 0.001). All patients had preserved LCA and MAP indices higher than 0.4 before IMA clamping. In 6 cases (13.0%), a critically low MAP index of less than 0.4 was noted after IMA clamping, which could be considered the indicator of potential colonic hypoperfusion (Fig. 3).
On the regression analysis, MAP indices showed a significant negative correlation with the atherosclerotic risk score (P = 0.004). As the atherosclerotic risk score increased, MAP indices decreased after IMA clamping (Fig. 4). The MAP indices and IMA length also revealed a significant positive correlation (P = 0.019).
On the univariate analysis, a critically low MAP index was associated with high atherosclerotic risk (P = 0.039) and shorter IMA length (P = 0.041). The critically low MAP index was more frequent in patients with a history of ischemic heart disease; however, this was not statistically significant (33.3% vs. 11.6%, P = 0.280). The presence of IMA atherosclerotic calcification was not significantly associated with a low MAP index (P = 0.655). Multivariate analysis indicated that high atherosclerotic risk and IMA length were independent clinical predictors of critically low MAP index reflecting hypoperfusion status after IMA clamping (Table 3).
IMA ligation remains a potential risk factor for hypoperfusion of the left-sided colon during rectosigmoid cancer surgery [6]. Following IMA ligation, colonic ischemia may occur, if the IMA stump pressure decreases to 40 mmHg or the mean IMA/systemic BP ratio was less than 0.4, which is considered the critical closing pressure during aortic aneurysm surgery [15]. A critically low MAP index of less than 40% of the systemic artery pressure was considered to present a high risk of colonic ischemia [15]. However, another study did not identify the significance of IMA index for predicting anastomotic leakage after left colon resection [16]. In another study, a marginal artery BP of 45 mmHg was also used as the cutoff value for insufficient colon perfusion [17].
To adjust the physiological differences of systemic BP in each patient, we adopted the MAP index: that is, the mesenteric marginal MAP was divided by the systemic MAP as the measurable outcome of critical hemodynamic change. In previous studies, the MAP ratio was calculated by measuring the IMA stump pressure [1516]. In this study, the mesenteric BP was measured by preserving the marginal artery nearest to the colon segment for anastomosis. Therefore, since mesenteric marginal artery pressure is generally lower than the IMA stump pressure, the application of the same cutoff for MAP index (<0.4) as in previous studies has the potential to overestimate the critical hypoperfusion.
In this study, most patients showed an immediate decrease of mesenteric BP within various deviations after IMA ligation. Fortunately, almost 90% of rectosigmoid cancer patients had mesenteric BP above the critical level after IMA ligation, but 13% of rectosigmoid cancer patients had a critically low MAP index after IMA ligation. This incidence of transient hypoperfusion after IMA ligation was similar to that of a previous study using laser Doppler assessment [18]. This could be related to the adaptive vasodilatation of the mesenteric circulation and considered a temporary change in most cases. Therefore, there is still controversy about the opinion that acute change in mesenteric perfusion could indicate the occurrence of anastomotic leakage. Nevertheless, continuous monitoring of mesenteric arterial flow restoration over several postoperative days is difficult in real practice, so to date, poor perfusion of mesenteric artery during surgery could be considered a potential risk factor for colonic ischemia.
A critically low MAP index was significantly correlated with the atherosclerotic risk score and IMA length on CT angiography in this series. Atherosclerosis is less likely to occur in small vessels, such as the mesenteric marginal artery [19]. Although there was no definite atherosclerotic change in the marginal artery on CT angiography, atherosclerotic degeneration-induced mesenteric microangiopathy could disturb the meticulous vascular accommodation of colonic mesentery following IMA ligation [20]. Therefore, atherosclerotic microangiopathy could have a negative effect on the adaptation of the mesenteric collateral circulation after IMA clamping, especially in patients with a high atherosclerotic risk.
Interestingly, the shorter the IMA length, the lower the MAP index after IMA clamping. In recent studies, the LCA blood flow from the SMA through the accessory MCA was considered the predominant blood flow when the IMA was long, and the blood flow from the IMA to the LCA had more dominant collateral circulation when the IMA was short [21]. For patients with an accessory MCA, blood flow through the SMA may be more prevalent in the mesenteric circulation, thus leading to safer IMA ligation. However, in the absence of an accessory MCA or intermesenteric artery, the mesenteric blood flow leading to the LCA from the IMA appears to be more important in the maintenance of perfusion status of the left colon [22]. In this study, the IMA length was revealed as an independent predictor of critically low MAP index after IMA clamping. Therefore, if a shorter IMA is detected on preoperative CT angiography or in the intraoperative field, it is necessary to consider low ligation of IMA or confirm that backflow is well maintained when high ligation is performed on the root of the IMA.
Atherosclerotic calcification of the major mesenteric arteries was not associated with mesenteric marginal MAP reduction after IMA clamping. As atherosclerosis progresses slowly, adaptation to chronic ischemia may continue without clinical symptoms if the patient maintains adequate cardiovascular function. The mesenteric blood flow of the left colon could be maintained by the collateral circuit from the SMA through the intermesenteric artery or marginal artery of Drummond [23]. This prevents a sudden decrease in mesenteric marginal MAP after IMA clamping in patients with mesenteric artery calcifications [24]. An alternative explanation is that arterial wall calcification could occur predominantly in the media and that exophytic growth, especially in chronic kidney disease, could have failed to induce mesenteric arterial stenosis [25]. Conversely, noncalcified atheroma formation is predominantly detected in the intima and is likely to cause stenotic changes, which increases the arterial flow disturbance [25].
In recent studies, SMA calcification, cardiovascular disease, and chronic kidney disease have been identified as important predictive factors of small bowel vascular lesions [26]. In this study, the incidence of critically low MAP was higher in patients with concomitant cardiovascular diseases of hypertension and ischemic heart disease; however, no statistical differences were observed.
The absence of marginal arcade on splenic flexure was found in 5%–10% of the general population, which could be interpreted as sufficient mesenteric collateral circulation in the left colon [10]. Therefore, critically low MAP indices could be due to a number of causative factors, such as stenotic atherosclerosis of the major mesenteric arteries, atherosclerotic microangiopathy of the marginal artery, or insufficient mesenteric collateral circulation.
This study had several limitations. First, clear evidence on defining a safe hemodynamic range for the marginal artery that can maintain adequate mesenteric blood flow to prevent ischemic colitis is lacking [151617]. In real practice of left colon resection, anastomosis is performed after complete mesenteric division from the IMA to the colonic transection line including the main vessel and the lymph node. Because previous studies considered that the marginal artery near the anastomotic colonic segment was expected to the best point that reflects the actual hypoperfusion state of the anastomotic colon segment, we measured the marginal artery pressure instead of the IMA stump pressure. Previous studies using IMA stump pressure and systemic artery pressure used 0.4 as a cut-off value for classification of MAP ratio. However, since marginal artery pressure has not been sufficiently studied, the previous criteria was adopted in the present study. However, because the marginal artery has smaller diameter than IMA, marginal artery pressure is generally lower than the IMA stump pressure. The cutoff value (<0.4) of MAP index has the potential to overestimate the critical hypoperfusion state, which is the limit of MAP index in this study.
Second, it was relatively difficult to evaluate details of mesenteric vascular atherosclerotic change, such as noncalcified, stenotic atheroma, endophytic, or exophytic growth of mesenteric arterial atherosclerosis and marginal vascular arcades on the splenic flexure using conventional CT angiography [2728]. Third, the blood flow is affected not only by pressure but also by resistance of the vessel, and the mesenteric BP may not consistently correlate with insufficient mesenteric perfusion [29]. Therefore, quantitative perfusion assessments could be considered in patients with high atherosclerotic risk or short IMA on CT angiography using intraoperative near-infrared fluorescence with indocyanine green [430]. Finally, this study included a small sample size and was performed at a single institution. Therefore, further large-scale multicenter trials are needed to verify our results.
In conclusion, atherosclerotic risk assessment and IMA length can be considered useful predictors of the mesenteric hypoperfusion status after IMA ligation during laparoscopic rectosigmoid colon surgery. Therefore, intraoperative colonic perfusion assessments should be considered, especially in patients with high atherosclerotic risk and short IMA detected on CT angiography.
Figures and Tables
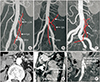 | Fig. 1CT angiography evaluation of mesenteric vascular properties. Inferior mesenteric artery (IMA) branching types; left colic artery (LCA) first (A), superior rectal artery (SRA) first (B), LCA, SRA, and sigmoidal artery branching simultaneously (C). (D) Internal diameter of IMA. (E) Intermesenteric artery. (F) Atherosclerotic calcification of IMA orifice. |
 | Fig. 2(A) Lymph node dissection was continued along the inferior mesenteric artery (IMA) to first bifurcation point and the branches of the IMA were identified. The superior rectal artery (SRA) and sigmoid artery were ligated while preserving the left colic artery. (B) After the transection of proximal colon, the remaining colonic mesentery was divided to leave a marginal artery and a 24-gauge intravascular catheter was cannulated to measure mesenteric arterial blood pressure. |
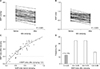 | Fig. 3Hemodynamic changes in the marginal artery according to inferior mesenteric artery (IMA) clamping. (A) The mean arterial pressure (MAP) alterations of the marginal artery before and after IMA clamping. (B) MAP index changes after IMA clamping. (C) Critically low MAP index after clamping (<0.4). (D) Histogram of MAP index after clamping. |
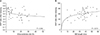 | Fig. 4Curve estimation regression graphs for critical hemodynamic alteration and predictive factors. (A) MAP index after clamping is significantly associated with the atherosclerotic risk score and (B) inferior mesenteric artery (IMA) length. |
Table 3
Clinical factors associated with mesenteric artery calcification and critical hemodynamic alteration

Values are presented as number (%) or mean ± standard deviation unless otherwise indicated.
OR, odds ratio; CI, confidence interval; MAP, mean arterial pressure; ASA PS, American Society of Anesthesiologists physical status; TC, total cholesterol; IMA, inferior mesenteric artery; SMA, superior mesenteric artery; LCA, left colic artery; MCA, middle colic artery.
ACKNOWLEDGEMENTS
The authors would like to thank the operation team who are involved in hemodynamic measurements in the surgical field of Pusan National University Yangsan Hospital.
References
1. Rao X, Zhang J, Liu T, Wu Y, Jiang Y, Wang P, et al. Prognostic value of inferior mesenteric artery lymph node metastasis in cancer of the descending colon, sigmoid colon and rectum. Colorectal Dis. 2018; 20:O135–O142.

2. Lee KH, Kim JS, Kim JY. Feasibility and oncologic safety of low ligation of inferior mesenteric artery with D3 dissection in cT3N0M0 sigmoid colon cancer. Ann Surg Treat Res. 2018; 94:209–215.

3. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013; 257:665–671.
4. Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc. 2019; 33:1640–1649.

5. Sindhu RS, Natesh B, Rajan R, Shanavas K, Sukumaran G, Gayathri LK. Low-tie IMA and selective D3 lymph node sampling in laparoscopic rectal resection for carcinoma rectum: comparison of surgical and oncological outcomes with the open technique. J Gastrointest Oncol. 2017; 8:850–857.

6. Kachlik D, Baca V. Macroscopic and microscopic intermesenteric communications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006; 150:121–124.

7. Fujii S, Ishibe A, Ota M, Suwa H, Watanabe J, Kunisaki C, et al. Short-term and long-term results of a randomized study comparing high tie and low tie inferior mesenteric artery ligation in laparoscopic rectal anterior resection: subanalysis of the HTLT (High tie vs. low tie) study. Surg Endosc. 2019; 33:1100–1110.

8. Miyamoto R, Nagai K, Kemmochi A, Inagawa S, Yamamoto M. Three-dimensional reconstruction of the vascular arrangement including the inferior mesenteric artery and left colic artery in laparoscope-assisted colorectal surgery. Surg Endosc. 2016; 30:4400–4404.

9. Champagne BJ, Lee EC, Valerian B, Mulhotra N, Mehta M. Incidence of colonic ischemia after repair of ruptured abdominal aortic aneurysm with endograft. J Am Coll Surg. 2007; 204:597–602.

10. Brandt LJ, Feuerstadt P, Longstreth GF, Boley SJ. American College of Gastroenterology. ACG clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol. 2015; 110:18–44.

11. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117:743–753.
12. Jahangiry L, Farhangi MA, Rezaei F. Framingham risk score for estimation of 10-years of cardiovascular diseases risk in patients with metabolic syndrome. J Health Popul Nutr. 2017; 36:36.

13. Ke J, Cai J, Wen X, Wu X, He Z, Zou Y, et al. Anatomic variations of inferior mesenteric artery and left colic artery evaluated by 3-dimensional CT angiography: insights into rectal cancer surgery - A retrospective observational study. Int J Surg. 2017; 41:106–111.

14. Hiltunen A, Kivisaari L, Leino-Arjas P, Vehmas T. Visual scoring of atherosclerosis in chest computed tomography: findings among male construction workers. Acta Radiol. 2008; 49:328–336.

15. Ernst CB, Hagihara PF, Daugherty ME, Griffen WO Jr. Inferior mesenteric artery stump pressure: a reliable index for safe IMA ligation during abdominal aortic aneurysmectomy. Ann Surg. 1978; 187:641–646.
16. Hsu TC. Inferior mesenteric artery stump pressure is an unreliable predictor of the outcome of colorectal anastomosis. Int J Colorectal Dis. 2007; 22:1481–1484.

17. Guo Y, Wang D, He L, Zhang Y, Zhao S, Zhang L, et al. Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: a clinical trial. ANZ J Surg. 2017; 87:576–581.

18. Seike K, Koda K, Saito N, Oda K, Kosugi C, Shimizu K, et al. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis. 2007; 22:689–697.

19. Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: similarities and differences. Prog Cardiovasc Dis. 2007; 50:112–125.

20. Tilsed JV, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016; 42:253–270.

21. Murono K, Kawai K, Kazama S, Ishihara S, Yamaguchi H, Sunami E, et al. Anatomy of the inferior mesenteric artery evaluated using 3-dimensional CT angiography. Dis Colon Rectum. 2015; 58:214–219.

22. Miyake H, Murono K, Kawai K, Hata K, Tanaka T, Nishikawa T, et al. Evaluation of the vascular anatomy of the left-sided colon focused on the accessory middle colic artery: a single-centre study of 734 patients. Colorectal Dis. 2018; 20:1041–1046.

23. Tasse JC, Arslan B, Turba UC. Isolated stenosis of the inferior mesenteric artery: to treat or not to treat? Tech Vasc Interv Radiol. 2015; 18:51–55.

24. Eikendal AL, den Ruijter HM, Haaring C, Saam T, van der Geest RJ, Westenberg JM, et al. Sex, body mass index, and blood pressure are related to aortic characteristics in healthy, young adults using magnetic resonance vessel wall imaging: the AMBITYON study. MAGMA. 2018; 31:173–182.

25. Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013; 93:365–373.

26. Aoyama T, Fukumoto A, Shigita K, Asayama N, Mukai S, Nagata S. Arteriosclerosis is a major predictor of small bowel vascular lesions. Dig Dis Sci. 2018; 63:723–730.

27. Burrill J, Dabbagh Z, Gollub F, Hamady M. Multidetector computed tomographic angiography of the cardiovascular system. Postgrad Med J. 2007; 83:698–704.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download