DISCUSSION
This second biannual report based on KOTRY registry data detailed trends regarding donor and recipient characteristics, post-transplant comorbidities and outcomes. We also focused on age in relationship to pre and post-transplant characteristics and outcomes. Since the initial report of the KOTRY registry was published, some distinct trends in HTx were noted despite the short-term follow-up duration. First, usage of mechanical support devices before transplant has significantly increased. Significantly more patients are on ECMO and ventilators in recent years compared to 2014–2015, indicating that more patients are getting transplanted when they are severely ill, most likely due to organ shortages. Second, in terms of age, the mean donor age increased significantly in the most recent year compared to 2014–2015, while the mean recipient age was unchanged.
The number of patients bridged with ECMO before HTx has increased in recent years. Both short- and intermediate-term survival was worse in pre-transplant ECMO-supported patients compared to those without pre-transplant ECMO. Further, patients with ECMO and ventilator support before transplant did far worse than those with pre-transplant ECMO without a ventilator. Previous data from the United Network of Organ Sharing thoracic registry reported post-transplant survival in patients who were bridged with ECMO to be 73% at 3 months and 67% at 3 years.
6) Corresponding survival rates from the KOTRY registry were 85% at 3 months and 77% at 3 years. The current standard treatments for bridging to transplant before HTx in the United States include durable ventricular assisting devices that yield better survival rates than ECMO.
3) Durable ventricular assisting devices are still not affordable for most patients in Korea, and so very few (n=5, 1%) had a ventricular assisting device as a bridge to transplant in the KOTRY data. This is a unique and distinct characteristic of current HTx practice in Korea compared to other countries where the use of ECMO as a direct bridge to transplantation in adults is very infrequent; the most recent International Society for Heart and Lung Transplant (ISHLT) registry reported that 1% of cases used ECMO as a bridge to transplant.
7) Relatively better survival in patients who were bridged with ECMO in the KOTRY data compared to other studies may result from a recent pre-emptive ECMO strategy, where patients undergo ECMO insertion electively rather than in an emergent setting. However, pre-transplant ECMO was still significantly associated with worse early and long-term survival outcomes in the KOTRY data. Unlike a durable ventricle assisting device, which allows patients to maintain a functional status, patients on ECMO support are immobilized in intensive care units with invasive monitoring and exposed to complications related to the ECMO and intensive care unit environment. Due to a shortage of organs and unaffordable durable ventricular supporting devices, more patients were bridged with ECMO before HTx. The major concern for the current practice in Korea includes poor post-transplant survival by preferentially allocating hearts to ECMO-supported patients.
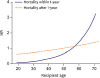
We focused on age in this second official KOTRY HTx report. With the development of mechanical devices to bridge to HTx and the increasing prevalence of heart failure among the elderly, the number of potential HTx candidates of advanced age is increasing. With organ shortages, age is an important issue for recipient and donor selection. The ISHLT guideline was modified in 2016 to address issues of HTx in patients with advanced age.
8) Previous studies have consistently reported comparable long-term survival outcome in patients with advanced age,
9)10)11) and so the class IIb recommendation accommodated consideration of patients older than 70 years for HTx after careful selection.
8) According to the 30th report from ISHLT, the proportion of recipients with age ≥70 in the 2006–2012 era was 1.3%. From the KOTRY data covering 2014–2017, the proportion of recipients age ≥70 who underwent HTx rose slightly to 2.8% (n=11).
12)
The majority of HTxs in recipients of advanced age (≥65) are performed in North America, and reports from high volume centers in North America show comparable long-term survival rates in recipients of advanced age (10-year survival for age ≥70, 60–69 years, ≤60 years was 51.7%, 47.7%, and 57.1%, respectively).
13) In contrast, the 30th ISHLT report showed worse survival with increasing recipient age.
12) Despite conflicting results, the current consensus is that age older than 70 years alone should not be an exclusion criterion for HTx. According to single center data reporting favorable outcome following HTx in patients older than 70, the age group of ≥70 had less comorbidities compared to transplant recipients in their 60s, suggesting that a stringent selection process is important for favorable outcomes in patients of advanced age. The KOTRY data analyzed according to year revealed that the proportion of HTx recipients age ≥70 has recently declined (n=6, 3% in 2014–2015; n=4, 4% in 2016; and n=1, 1% in 2017). This trend reflects the current practice of thorough patient selection in advanced age patients as HTx candidates in Korea. Newly emerged alternative treatment choices, such as LVADs for destination therapy, may also have contributed to this trend.
Due to the relatively short follow-up duration, no differences in post-transplant comorbidities or causes of death among different age groups were noted in the KOTRY data, although previous studies suggest that post-transplant comorbidities and causes of death differ according to recipient age. In ISHLT data, death from graft failure, including coronary allograft vasculopathy and acute rejection, decreased with increasing age, while death related to multiple organ failure, infection and non-lymphoma malignancy increased.
8) Declining immuno-competency with aging is known to potentially lower rejection rates due to lower generation of new T and B lymphocytes, which also contributes to increasing comorbidities associated with cancer and infection.
14)15) Different immuno-competency with aging raises questions about whether post-transplant screening and management for rejection or comorbidities should differ according to age group, yet data remain insufficient to answer these questions.
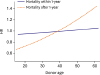
Clearly, donor age increased in Korea from 2014 to 2017, over a relatively short period of time. Previous data from ISHLT also describe the same trends of increasing donor age.
8) The median donor age in the KOTRY registry was 42, which was similar to the donor age in Europe (median age of 43), and older than that in North America (median age of 29). Increasing demand for donor organs has inevitably expanded the donor pool to include so-called marginal donors. A previous retrospective study analyzing outcome of 228 HTxs suggested that having a marginal donor (>50 years) did not affect 1,3, or 5 year mortality if associated with a short ischemic time, however, patients with older donors presented with more frequent coronary allograft vasculopathy.
16) The increasing incidence of coronary allograft vasculopathy with increasing donor age might be explained by age-related endothelial dysfunction and age correlated increases in the incidence of pre-existing coronary artery disease of donor hearts.
17) Data from the 30th report from ISHLT showed that increasing donor age was associated with worse survival, particularly with donors aged ≥60. Although younger donor age was accepted as an important prognostic factor for favorable long-term outcome after HTx, it remains worthwhile to consider accepting marginal donors, because the survival of patients who receive a transplant from a selected marginal donor might still be better than for those who were not transplanted at all.
16) Consequently, there is increasing acceptance for marginal donors with careful selection, such as short ischemic time, and this trend was reflected in the KOTRY data.
In conclusions, KOTRY, the nationwide organ transplantation database supported by the Korean government, was established in an effort to provide evidence for a national organ transplant policy. Using KOTRY data, this report provided a comprehensive analysis of current transplantation data in Korea. Over 4 years, with increasing organ shortages, centers were more willing to accept older age donors, and more patients received transplants under ECMO care. Increasing age was a strong independent factor for intermediate long-term survival, but post-transplant comorbidities did not differ among age groups, possibly due to the relatively short-term follow-up period. Further study with longer follow-up duration is needed to better understand age-related post-transplant prognosis.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download