INTRODUCTION
MATERIALS AND METHODS
Study population
Clinical findings
Thoracic radiography
Clinical outcomes
Echocardiographic measurements
RPA measurements
 | Fig. 1M-mode echocardiographic images of the right pulmonary artery.(A) The right parasternal four-chamber long-axis view was interrogated by the 2-dimensional image system. The right pulmonary artery is indicated by a white arrow. The systolic (S) and diastolic (D) diameters (generally at the T wave and Q wave, respectively) were measured using the “leading edge-to-leading edge” technique. The RPAD index was calculated from the difference between S and D divided by S. The dicrotic notch of the right pulmonary artery (arrow) in a normal dog (B) and in a dog with severe pulmonary hypertension caused by heartworm disease (C).
RPAD, right pulmonary artery distensibility
|
Inter-observer and intra-observer measurements
Statistical analysis
RESULTS
Study population and clinical findings
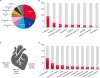 | Fig. 2The characteristics of study population (n = 200). (A) Breed included in our study; the Maltese, mix and Chihuahua were top 3. (B) Clinical signs distribution, 1 dog could have more than one sign; the most common clinical sign was coughing. (C) The point of maximal intensity of heart murmur, which was not detected in 29% of cases. (D) The thoracic radiography was available in 190 dogs; the most common finding was cardiomegaly.LAE, left atrial enlargement; MPA, main pulmonary artery; PA, pulmonary artery; RVE, right ventricular enlargement.
|
TRPG group
Table 1
Differences in variables between dogs based on TRPG and diagnosis

Findings according to diagnosis
Correlation analysis
Table 2
Correlations between clinical assessments and echocardiographic variables

 | Fig. 3Scatter plots for the indices. Scatter plot and regression line for RPAD and TRPG (A) and AT/ET and TRPG (B). The TRPG and RPAsn (C) and RPAdn (D).AUC, area under the curve; RPAD, right pulmonary artery distensibility; TRPG, peak tricuspid regurgitation systolic pressure gradient; AT/ET, ratio of acceleration time to ejection time of pulmonary artery; RPAsn, normalized end-systolic right pulmonary artery dimension; RPAdn, normalized end-diastolic right pulmonary artery dimension.
|
Diagnostic accuracy and optimal cut-off values for predicting PH
Table 3
Statistical analysis of ROC curves for variables that predict TRPG ≥36 mmHg and TRPG ≥50 mmHg

Survival analysis
 | Fig. 4Receiver-operating characteristic curves for the indices. Receiver-operating characteristic curves for TRPG, RPAD, and RPAsn, and RPAdn that predict the 3-month (A) and 1-year (B) survival in dogs.TRPG, peak tricuspid regurgitation systolic pressure gradient; RPAD, right pulmonary artery distensibility; AT/ET, ratio of acceleration time to ejection time of pulmonary artery; RPAsn, normalized end-systolic right pulmonary artery dimension; RPAdn, normalized end-diastolic right pulmonary artery dimension.
|
 | Fig. 5Kaplan-Meier survival curves for the indices. Kaplan-Meier survival curves for TRPG, RPAD, RPAsn, and RPAdn at 3 months (A) and 1 year (B).RPAD, right pulmonary artery distensibility; TRPG, peak tricuspid regurgitation systolic pressure gradient; RPAsn, normalized end-systolic right pulmonary artery dimension; RPAdn, normalized end-diastolic right pulmonary artery dimension.
|
Table 4
Survival time in the groups with 3 months and 1 year of follow-up

Table 5
Binary logistic regression analysis of pulmonary hypertension-related deaths during 3 months of follow-up and 1 year of follow-up





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download