Abstract
Actinomycosis can mask malignant diseases. This paper reports a case of colonic diffuse large B-cell lymphoma (DLBCL), which was misdiagnosed as abdominal actinomycosis. A 76-year-old woman presented with right flank pain and weight loss. Abdominal CT and colonoscopy revealed a huge ascending colon mass. Despite the initial impression of a malignancy, a colonoscopic biopsy revealed no malignant cells, but sulfur granules and a filamentous organism suggesting actinomycosis. Intravenous penicillin G was administered under the impression of abdominal actinomycosis but her condition deteriorated rapidly. Follow up CT showed markedly increased colon mass and new multiple nodular lesions around the ascending colon. Sono-guided percutaneous biopsy of the nodular lesion was performed. The pathological result was DLBCL. The patient was scheduled to undergo chemotherapy but the patient expired due to cancer progression. The diagnosis of gastrointestinal infiltrating tumors is often difficult because a superficial biopsy usually does not provide a confirmative diagnosis. This case highlights the difficulty in making a correct diagnosis of lymphoma due to the concomitant actinomycosis. Malignant conditions must be considered in cases of actinomycosis with no response to antimicrobial therapy.
Lymphoma originating from the gastrointestinal (GI) tract comprises 5–20% of the total lymphoma cases and 0.5% of malignant tumors of the GI tract. The stomach is the most frequent site of GI lymphoma. Lymphoma is found less commonly in the small and large intestine.12 GI lymphoma is often difficult to diagnose by a routine endoscopic biopsy, which requires a deep biopsy.3 The diagnosis and treatment of colonic actinomycosis are often difficult because the clinical features and radiological findings are similar to malignant tumors, which is more prevalent.4 The paper reports a case of colonic diffuse large B-cell lymphoma (DLBCL) for which the diagnosis was delayed due to actinomycosis that accompanied the tumor along with a review of the relevant literature.
A 76-year-old female who had suffered from weight loss and a febrile sense for two months visited the emergency room complaining of right lower quadrant pain with a mass. She had been taking medication for hypertension and diabetes. Measurements of the vital signs were as follows: blood pressure, 135/52 mmHg; pulse rate, 89 beats per minute; respiratory rate, 20 breaths per minute; and body temperature, 36.3℃. A physical examination revealed a tender round mass in the right lower quadrant of the abdomen. No significantly enlarged lymph nodes were observed at the groin, neck, and axilla. A laboratory examination showed the following: leukocytes, 10,000/mm3; hemoglobin, 7.1 g/dL; platelets 525,000/mm3; BUN, 31.3 mg/dL; creatinine, 1.5 mg/dL; LDH, 772 U/L; CRP, 4.058 mg/dL; total bilirubin, 0.3 mg/dL; aspartate aminotransferase, 26 IU/L; alanine aminotransferase, 19 IU/L; sodium, 133 mEq/L; potassium, 5.1 mEq/L; and chloride, 103 mEq/L. Abdominal CT showed a huge mass with enlarged lymph nodes around the ascending colon; the mass was assumed to invade the right kidney (Fig. 1).
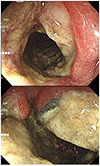
For a further evaluation of the colonic mass, a colonoscopy was performed, which revealed an ulcerative mass accompanied by a luminal stricture at the hepatic flexure and ascending colon (Fig. 2). Multiple biopsy was performed, and a histology examination revealed inflammatory necrotic tissues without neoplastic cells (Fig. 3A). Filamentous bacterial colonies, which were morphologically compatible with an Actinomyces species, were found (Fig. 3B).
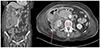
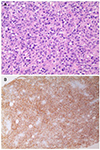
Under the impression of abdominal actinomycosis, she received intravenous penicillin (penicillin G 15 million units/day in six divided doses, every 4 hours). On the other hand, two weeks of a penicillin injection did not improve the abdominal pain and mass, and the patient's condition worsened. Follow up abdominal CT was performed, which showed an increase in the size of the mass and the additional enlargement of lymph nodes other than those previously found (Fig. 4). This led to the assumption of a misdiagnosis of this patient, and sono-guided percutaneous fine needle biopsy was performed for right retroperitoneal mass to obtain deep and sufficient tissue material. A histopathologic examination of the biopsy specimens showed sheets of large pleomorphic lymphocytes with prominent nucleoli and occasional apoptotic bodies (Fig. 5). The immunohistochemical study showed that the tumor cells were positive for CD20 (Fig. 5), CD79a, CD5, Bcl-6, Bcl-2, and MUM-1, but negative for pan-cytokeratin, CD3, and CD10. The tumor cells showed a high Ki-67 labeling index (≥90%). These pathology features were compatible with high grade B-cell lymphoma, and the patient was diagnosed with colonic DLBCL, Lugano IIE with an international prognostic index score 3. She was scheduled for chemotherapy, but her general condition deteriorated rapidly due to the rapid progression of DLBCL, and the patient expired a few weeks later.
Lymphoma is classified as primary if there is no invasion into the peripheral or mediastinal lymph nodes, no enlargement of the liver or spleen, normal white blood cell counts, no spread to remote lymph nodes but restricted to the GI tract and regional lymph nodes.5 Primary malignant lymphoma of the colon and rectum is rare, most of which occurs at the cecum (73%), followed by the ascending colon and rectum.6 The reason why more primary lymphoma cases are observed frequently at the cecum was assumed to be related to its larger amount of lymphoid structure.7 Recent trends show an increase in primary lymphoma in the GI tract; this is assumed to be because of the increase in the number of patients suffering from acquired or iatrogenic immunodeficiency due to a range of conditions, such as inflammatory bowel disease, human immunodeficiency virus infections, and organ transplantation.8 Malignant lymphoma is classified as B cell and T cell lymphoma according to the revised European-American lymphoma classification. B-cell lymphoma generally forms a mass and can be misdiagnosed as adenocarcinoma of the colon. In particular, DLBCL tends to involve the long segment of the intestine and forms a large mass that accompanies an ulcer and stricture. On the other hand, T cell lymphoma generally leads to an ulcer but can be misdiagnosed as inflammatory diseases, such as inflammatory bowel disease, intestinal tuberculosis, or infectious colitis.9
Colonoscopy with a biopsy and abdominal CT scan are important tools for diagnosing primary lymphoma of the colon. Endoscopic morphology of colon lymphoma can be categorized into three types - fungating, ulcerative, and infiltrative - and two other mixed types - ulcerofungating and ulcer infiltrative.10 Fungating and ulcerofungating are the most common types, but it is difficult to distinguish these types of lymphoma from an adenocarcinoma. Some rare cases of multiple lymphomatous polyposis have also been encountered. A histology examination is critical for diagnosis. On the other hand, lymphoma can frequently be missed from a biopsy if the possibility of the disease has not been taken into consideration. In contrast to adenocarcinoma, GI lymphoma develops pathologically from the lymphoid follicle at the submucosal layer, which leads to the spread of disease mainly within this layer, making it difficult to diagnosis by a mucosal biopsy.11 An abdominal CT examination may show a degree of infiltration by the tumor or enlargement of regional lymph node.12
Actinomycosis is a chronic suppurative granulomatous disease caused by an anaerobic or microaerobic strain that normally is found in the mouth, colon, and female genitals; the most common strain is Actinomyces israelii. Abdominal actinomycosis is generally caused by abdominal surgery or external injury, but the disease can also develop in patients who have been treated with steroids for a long time, chemotherapy for leukemia, or renal transplant, all of which may result in damage to the mucosal layer.13 For this specific case, it was assumed that actinomycosis developed from a damaged colon mucosal layer caused by lymphoma. A diagnosis actinomycosis may be difficult because a clinical and radiological manifestation of the disease may mimic that of a malignant tumor. Abdominal actinomycosis can be considered if a heterogeneous abdominal mass infiltrates through the serous membrane and shows fewer invasions into the lymph nodes compared to what is expected from the lesion size.14
Several cases similar to this one have been published. One is gastric DLBCL, which was first misdiagnosed as gastric actinomycosis by endoscopic biopsies.15 In this case, a persistent malignant appearing ulcer despite antimicrobial therapy was the clue of a misdiagnosis and the patient underwent a surgical resection revealing DLBCL, followed by adjuvant chemotherapy. In addition, there are several cases of colon actinomycosis mimicking cancer, leading to an unnecessary surgical resection of a benign lesion.16 On the other hand, there is no case report of a colonic malignancy misdiagnosed as actinomycosis. This case emphasizes the importance of considering a possible hidden malignancy when abdominal actinomycosis is treated without surgery, particularly in cases of no response on effective antibiotics.
In conclusion, the final diagnosis in this case was colonic DLBCL, which was misdiagnosed as abdominal actinomycosis. The diagnosis of GI lymphoma may be difficult by an endoscopic biopsy alone, and can be obscured by actinomycosis, as in this case. If an actinomycotic mass that does not respond to effective antibiotics is encountered, a hidden malignant tumor must be considered, which require a repeat biopsy by various modalities (e.g., fine needle aspiration or open biopsy) for a confirmatory diagnosis.
Figures and Tables
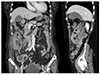 | Fig. 1Abdominal computed tomography scan shows a huge mass at the ascending colon with nearby multiple enlarged lymph nodes. |
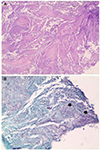 | Fig. 3(A) Histology examination showing inflammatory necrotic tissues (H&E, ×100). (B) Silver special staining revealing the filamentous bacterial colonies (arrows) (GMS, ×400). |
References
1. Andrews CN, John Gill M, Urbanski SJ, Stewart D, Perini R, Beck P. Changing epidemiology and risk factors for gastrointestinal non-Hodgkin's lymphoma in a North American population: population-based study. Am J Gastroenterol. 2008; 103:1762–1769.

2. Katsumata R, Matsumoto H, Motoyasu O, et al. Primary colorectal lymphoma comprising both components of diffuse large B-cell lymphoma and mucosa-associated lymphoid tissue lymphoma combined with cytomegalovirus colitis. Clin J Gastroenterol. 2016; 9:59–62.

3. Choi MK, Kim GH. Diagnosis and treatment of gastric MALT lymphoma. Korean J Gastroenterol. 2011; 57:272–280.

4. Lim JA, Wong PS, Leong KN, Wong KL, Chow TS. Masking and misleading: concomitant actinomycosis and B-cell lymphoma - a case report and review of literature. Scott Med J. 2018; 63:125–131.

5. Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001; 33:267–282.

6. Shepherd NA, Hall PA, Coates PJ, Levison DA. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1998; 12:235–252.

8. Kim YH, Lee JH, Yang SK, et al. Primary colon lymphoma in Korea: a KASID (Korean Association for the Study of Intestinal Diseases) study. Dig Dis Sci. 2005; 50:2243–2247.

9. Ryu SH, Cheon JH, Kim JY, et al. The early diagnostic accuracy for gastrointestinal T-cell lymphoma from a perspective of gastroenterologists. Intest Res. 2011; 9:19–26.

10. Choe WH, Kim YH, Kim BJ, et al. The different colonoscopic manifestations of primary colorectal lymphomas by their cellular origin. Intest Res. 2003; 1:22–30.
11. Jiang C, Gu L, Luo M, Xu Q, Zhou H. Primary rectal lymphoma: a case report and literature review. Oncol Lett. 2015; 10:43–44.

12. Lee HJ, Han JK, Kim TK, et al. Primary colorectal lymphoma: spectrum of imaging findings with pathologic correlation. Eur Radiol. 2002; 12:2242–2249.

13. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014; 7:183–197.
14. Ercolak V, Paydas S, Ergin M, et al. Abdominal actinomycosis with multiple myeloma: a case report. Oncol Lett. 2014; 8:1876–1878.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download