INTRODUCTION
Chronic empyema-associated malignancy (CEAM) is a rare complication of long-standing empyema. In a study of 134 patients in Japan, Iuchi et al. (
1) reported that the incidence of malignant tumors in the setting of a chronic empyema was 5.2%. In that study, three cases of non-Hodgkin's lymphoma were identified, and one case of each of the following: malignant mesothelioma, angiosarcoma, malignant fibrous histiocytoma, and adenocarcinoma. In addition, it was found that malignant lymphoma can develop in the pleural cavity of patients with long-standing inflammatory tuberculous pyothorax, particularly in those who have experienced an artificial pneumothorax (
2).
There have been only a few reports dealing with the radiological findings associated with CEAM. Minami et al. (
3) described the features of plain chest radiographs suggestive of malignancy, which included an increased opacity in the thoracic cavity, soft tissue bulging, reduced sharpness of the fat planes in the chest wall, destruction of bone near the empyema, extensive medial deviation of the calcified pleurae, and new occurrence of an air-fluid level in the empyema cavity. A more recent study investigated the accuracy of the computed tomography (CT) diagnostic criteria for CEAM in patients with chronic empyema. Lee et al. (
4) reported that the presence of a mass, involvement of the extrapleural fat, chest wall, and lung, nodular pleural thickening, mediastinal involvement, and separate lung nodules were significantly associated with CEAM. In addition, they used a logistic regression model to show that the presence of a mass and an empyema of the mediastinal pleura are significant predictors of CEAM, although these parameters generally had either low positive predictive value (PPV) or low sensitivity.
There has been only one report regarding the usefulness of
18F-fluorodeoxyglucose positron emission tomography/CT (
18F-FDG PET/CT) in patients with pyothorax-associated lymphoma (PAL) (
5). In that study, the authors concluded that
18F-FDG PET/CT was useful in several aspects of the PAL management: diagnosis, assessment of treatment efficacy, restaging, and follow-up. However, they did not suggest diagnostic criteria pertaining to
18F-FDG PET/CT for diagnosing CEAM in chronic empyema. To the best of our knowledge, there have been only four case reports describing the
18F-FDG PET/CT findings in CEAM (
6789), all of which described an intense
18F-FDG uptake only within the malignant portion of the empyema cavity. Furthermore, there have been no studies assessing the diagnostic performance of
18F-FDG PET/CT for differentiating CEAM from chronic empyema. Therefore, the purpose of this study was to retrospectively investigate the diagnostic performance of the
18F-FDG PET/CT criteria for diagnosing CEAM in patients with chronic empyema.
Go to :

MATERIALS AND METHODS
This retrospective, single-institution study was approved by our Institutional Review Board. The requirement for written consent was waived by the board.
Patients
Through a search of the electronic medical record database of our institution, we found 72 consecutive 18F-FDG PET/CT scans of 58 patients that included the term “chronic empyema” in the scan report. The following inclusion criteria were used to identify the final study population: 1) patients with CT findings typical of chronic empyema, or typical symptoms of chronic empyema at the time of the 18F-FDG PET/CT scan and 2) available histopathology results, or follow-up imaging studies of at least 6 months if histopathological confirmation was not available. Finally, 33 PET/CT scans of 33 patients were included in our study. The indications for 18F-FDG PET/CT were a suspicion for CEAM (n = 19) and a routine follow-up for other known malignancy (n = 14).
18F-FDG PET/CT Imaging
All subjects fasted for at least 6 hours and were verified to have blood glucose < 200 mg/dL at the time of the 18F-FDG injection. PET/CT scanning was performed using the Discovery LS (GE Healthcare, Milwaukee, WI, USA). A whole-body CT scan was performed with a continuous spiral technique using an 8-slice helical CT with a gantry rotation speed of 0.8 seconds. CT scan data were collected at 40–120 mAs adjusted to patient body weight, 140 keV, a section width of 5 mm, and a table feed of 5 mm per rotation. No intravenous or oral contrast material was used. After the CT scan, an emission scan was obtained from the thighs to the head, at 4 minutes per frame over 60 minutes, following an intravenous injection of up to 370 MBq 18F-FDG. Attenuation-corrected PET images from the CT data were reconstructed using an ordered-subsets expectation maximization algorithm (28 subsets and 2 iterations). The standardized uptake value (SUV) was calculated by adjusting for patient body weight and the actual dose of 18F-FDG. Commercial software (AW version 4.4, GE Healthcare) was used to co-register the separate CT and PET scan data.
Image Analysis and Definitions
The 18F-FDG PET images, non-contrast CT images, and fused 18F-FDG PET/CT images were reviewed by two nuclear medicine physicians (with a 15-years' and 7-years' experience, respectively), who were blinded to the results of other imaging examinations or the final histopathology results.
The following
18F-FDG PET/CT findings were analyzed by the reviewers in a consensus manner: 1) shape of the empyema cavity (oval, lenticular, or crescentic) (
10), 2) presence of fistula (air-fluid level in the empyema cavity) (
10), 3) maximum SUV within the empyema cavity, 4) uptake pattern of the empyema cavity (focal, with discrete hypermetabolic foci with activity significantly higher than that of the surrounding empyema cavity, versus non-focal, diffuse uptake without significant focal uptake), 5) presence of a protruding soft tissue mass within the empyema cavity, and 6) involvement of adjacent structures, including the ribs, chest wall, extrapleural fat, and lung. For analyzing the involvement of adjacent structures, contrast-enhanced CT scans were also reviewed. In order to maintain consistency during the analysis of the
18F-FDG PET/CT findings, the window level on the workstation monitor was set to a range of 0 to 5 SUVs.
In addition, further analysis was done to determine whether a combination of the significant 18F-FDG PET/CT diagnostic criteria could improve its diagnostic efficacy for detecting CEAM. We analyzed the following combinations: 1) high maximum SUV (> 10.8) and presence of a protruding soft tissue mass within the empyema cavity, 2) high maximum SUV within the empyema cavity and involvement of adjacent structures, 3) presence of a protruding softtissue mass within the empyema cavity and involvement of adjacent structures, and 4) high maximum SUV within the empyema cavity, presence of a protruding soft tissue mass within the empyema cavity, and involvement of adjacent structures
Final diagnosis was determined either by histopathology or by clinical follow-up. In the cases where the follow-up chest CT findings did not reveal any significant changes for more than 6 months, or showed a decrease in the extent of the chronic empyema, those findings were considered as benign.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical package, version 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were reported as mean ± standard deviation, and continuous variables were reported as median and range. Categorical variables were reported as frequency and percentage. Fisher's exact test and Mann-Whitney U test were used to compare the clinical and PET/CT findings in order to evaluate the ability of each criterion to differentiate CEAM from chronic empyema. A receiver operating characteristic (ROC) curve analysis was performed to assess the accuracy of the maximum SUV within the empyema cavity in differentiating benign chronic empyema from CEAM. The diagnostic accuracy was expressed as the area under the corresponding ROC curve. The cut-off points were the values that yielded the maximum value of the sum of sensitivity and specificity. The corresponding sensitivity, specificity, PPV, negative predictive value (NPV), and accuracy were calculated for each finding. McNemar's test and Fisher's exact test were used to compare the diagnostic performance between the significant 18F-FDG PET/CT features. A p value less than 0.05 was considered to indicate a statistically significant difference for all analyses.
Go to :

RESULTS
Of the 33 patients, 27 were men and 6 were women, with a mean age of 62.0 ± 9.5 years (age range, 45–81 years). Twenty-three patients had a past history of pulmonary tuberculosis or tuberculous pleuritis. Histopathological diagnosis was obtained in 14 patients. Six of the lesions were proven to be malignant, as follows: diffuse large B-cell lymphoma (n = 3), squamous cell carcinoma (n = 2), and poorly differentiated carcinoma (n = 1). The remaining eight lesions were histologically proven as benign, such as a chronic granulomatous inflammation. There was no clinical or radiologic evidence of CEAM in the remaining 19 patients during a clinical follow-up for at least six months.
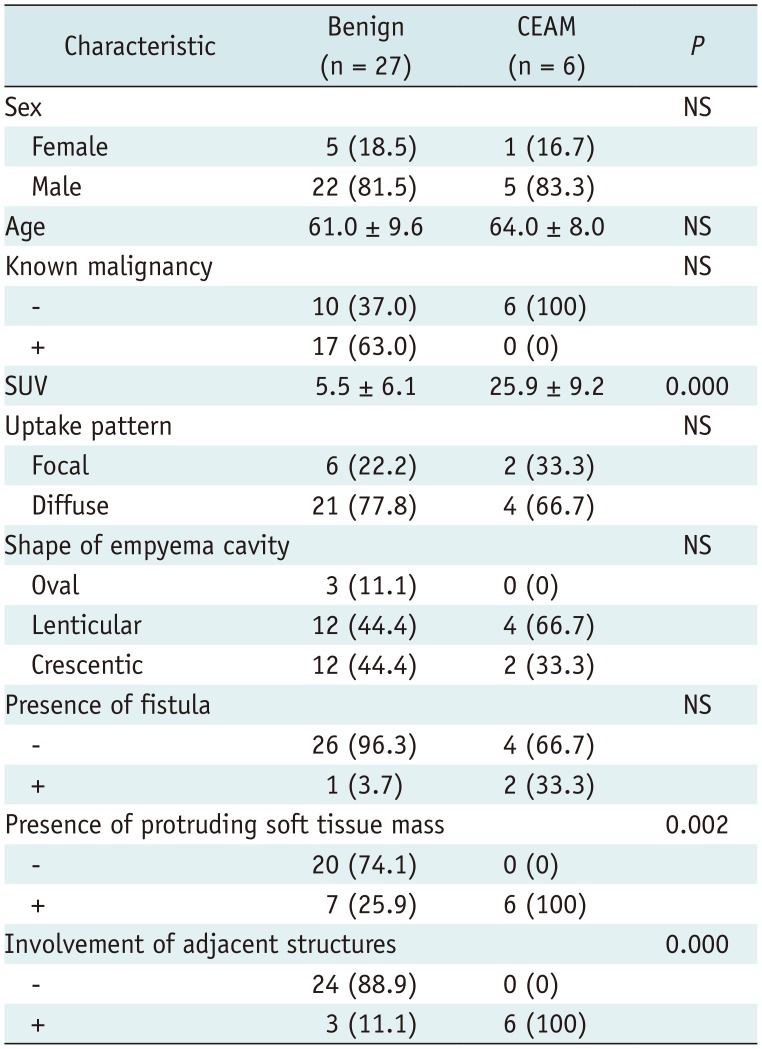
The
18F-FDG PET/CT findings in chronic empyema were compared with those in CEAM (
Table 1). In our study, the incidence of CEAM was 18.2% (6/33). The findings of maximum SUV of the empyema cavity, presence of a protruding soft tissue mass, and involvement of the adjacent structures in CEAM significantly differed from those in chronic empyema. In patients with CEAM, the maximum SUV was significantly higher than that in patients with chronic empyema. The presence of a protruding soft-tissue mass and involvement of the adjacent structures were more frequently observed in patients with CEAM than in those with chronic empyema. There were no significant differences in the empyema shape, presence of fistula, or uptake pattern of the empyema cavity between CEAM and chronic empyema. In addition, there was no significant linear correlation between the maximum SUV and the size of the empyema (
r = 0.331,
p = not significant, 0.06).
Table 1
Comparison of Clinical and PET/CT Findings between CEAM and Chronic Empyema
|
Characteristic |
Benign (n = 27) |
CEAM (n = 6) |
P
|
|
Sex |
|
|
NS |
|
Female |
5 (18.5) |
1 (16.7) |
|
|
Male |
22 (81.5) |
5 (83.3) |
|
|
Age |
61.0 ± 9.6 |
64.0 ± 8.0 |
NS |
|
Known malignancy |
|
|
NS |
|
- |
10 (37.0) |
6 (100) |
|
|
+ |
17 (63.0) |
0 (0) |
|
|
SUV |
5.5 ± 6.1 |
25.9 ± 9.2 |
0.000 |
|
Uptake pattern |
|
|
NS |
|
Focal |
6 (22.2) |
2 (33.3) |
|
|
Diffuse |
21 (77.8) |
4 (66.7) |
|
|
Shape of empyema cavity |
|
|
NS |
|
Oval |
3 (11.1) |
0 (0) |
|
|
Lenticular |
12 (44.4) |
4 (66.7) |
|
|
Crescentic |
12 (44.4) |
2 (33.3) |
|
|
Presence of fistula |
|
|
NS |
|
- |
26 (96.3) |
4 (66.7) |
|
|
+ |
1 (3.7) |
2 (33.3) |
|
|
Presence of protruding soft tissue mass |
|
|
0.002 |
|
- |
20 (74.1) |
0 (0) |
|
|
+ |
7 (25.9) |
6 (100) |
|
|
Involvement of adjacent structures |
|
|
0.000 |
|
- |
24 (88.9) |
0 (0) |
|
|
+ |
3 (11.1) |
|
|

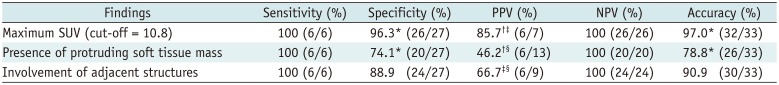
The diagnostic performance of each significant
18F-FDG PET/CT finding for diagnosing CEAM is presented in
Table 2. The maximum SUV within the empyema cavity was significantly higher in CEAM (25.9 ± 9.2) than in chronic empyema (5.5 ± 6.1) (
p < 0.001). On ROC curve analysis, the area under curve (AUC) of the maximum SUV was 0.994 (
Fig. 1). When a cut-off for maximum SUV was set at 10.8, the sensitivity, specificity, PPV, NPV, and accuracy for diagnosing CEAM were 100% (6/6), 96.3% (26/27), 85.7% (6/7), 100% (26/26), and 97.0% (32/33), respectively. The diagnostic performance of the maximum SUV was the highest among the significant
18F-FDG PET/CT features. In particular, the maximum SUV exhibited a significantly higher specificity, PPV, and accuracy than all other
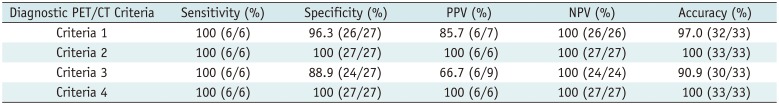
18F-FDG PET/CT findings. After comparison analysis using the combinations of
18F-FDG PET/CT diagnostic criteria, no significant differences were observed between the combined diagnostic criteria and neither of them exhibited a significantly greater diagnostic efficacy than that of the maximum SUV alone (all
p > 0.05) (
Table 3). Representative cases are illustrated in
Figures 2 and
3.
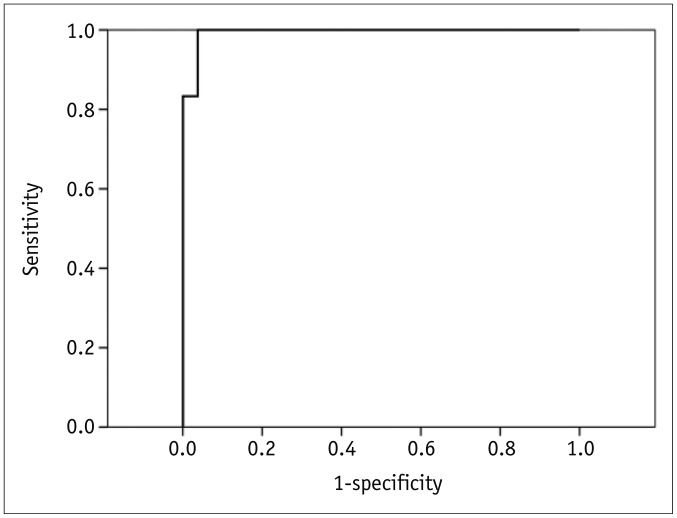 | Fig. 1
Receiver operating characteristic curve of maximum standardized uptake value for diagnosing chronic empyema-associated malignancy in patients with chronic empyema.
AUC was 0.994 ± 0.010 (p < 0.001). AUC = area under curve

|
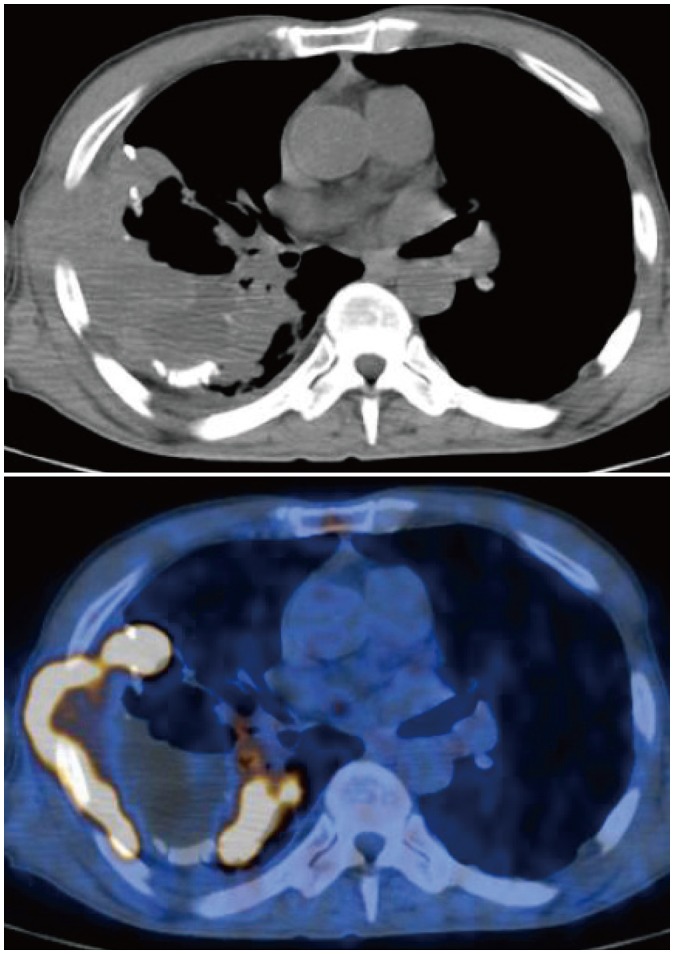 | Fig. 2
Non-contrast CT and fused PET/CT images of 68-year-old man with 40-year history of chronic tuberculous empyema.
Highly hypermetabolic lesion (maximum SUV = 29.6) involving adjacent chest wall is present within empyema cavity. Lesion was histopathologically proven as diffuse large B-cell lymphoma. CT = computed tomography, PET = positron emission tomography, SUV = standardized uptake value

|
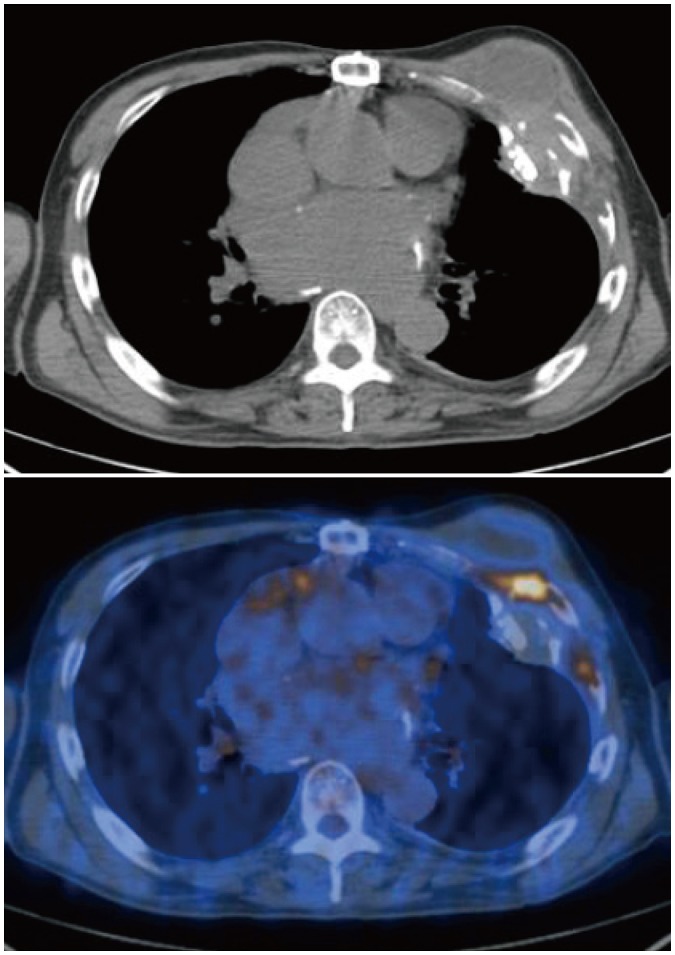 | Fig. 3
Non-contrast CT and fused PET/CT images of 69-year-old man with chronic empyema.
Moderately hypermetabolic lesion (maximum SUV = 7.0) involving adjacent rib is seen. Lesion was histopathologically proven as chronic active inflammation.

|
Table 2
Diagnostic Performance of PET/CT Findings for Diagnosing CEAM
|
Findings |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
|
Maximum SUV (cut-off = 10.8) |
100 (6/6) |
96.3* (26/27) |
85.7†‡ (6/7) |
100 (26/26) |
97.0* (32/33) |
|
Presence of protruding soft tissue mass |
100 (6/6) |
74.1* (20/27) |
46.2†§ (6/13) |
100 (20/20) |
78.8* (26/33) |
|
Involvement of adjacent structures |
100 (6/6) |
88.9 (24/27) |
66.7ठ(6/9) |
100 (24/24) |
90.9 (30/33) |

Table 3
Comparisons of Diagnostic Performance between Various Kinds of Diagnostic PET/CT Criteria for Differentiating CEAM from Chronic Empyema
|
Diagnostic PET/CT Criteria |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
|
Criteria 1 |
100 (6/6) |
96.3 (26/27) |
85.7 (6/7) |
100 (26/26) |
97.0 (32/33) |
|
Criteria 2 |
100 (6/6) |
100 (27/27) |
100 (6/6) |
100 (27/27) |
100 (33/33) |
|
Criteria 3 |
100 (6/6) |
88.9 (24/27) |
66.7 (6/9) |
100 (24/24) |
90.9 (30/33) |
|
Criteria 4 |
100 (6/6) |
100 (27/27) |
100 (6/6) |
100 (27/27) |
100 (33/33) |

Go to :

DISCUSSION
Although relatively rare, a malignant neoplasm arising from the pleural space in the setting of chronic inflammation is an important and critical complication of chronic empyema, because the management of these two conditions is quite different. Although the optimal management of CEAM has not been well determined, the overall prognosis in patients with PAL is known to be poor (
1112). In our study, the incidence of CEAM in patients with chronic empyema was 18.2% (6/33), which was higher than that reported in previous studies (
167). This may be due to the selective referral of patients with more serious conditions to our tertiary center, and the high prevalence of tuberculosis in Korea.
Establishing the diagnosis of CEAM based solely on the clinical symptoms and radiologic findings is challenging, particularly since an early detection by plain chest radiographs or chest CT remains difficult. Although several CT findings, such as the presence of a soft tissue mass and involvement of adjacent structures, may suggest malignancy, these findings are associated with either a low PPV or low sensitivity. Moreover, histopathologic confirmation is often difficult to obtain. Nevertheless, it is important to identify CEAM because it is a malignant and life-threatening condition with a prognosis much worse than that of benign chronic empyema.
In our study, we identified several useful
18F-FDG PET/CT findings for diagnosing CEAM in patients with chronic empyema, including the maximum SUV, presence of a protruding soft tissue mass, and involvement of adjacent structures. These results are consistent with those of Lee et al. (
4), who showed that the presence of a mass and involvement of adjacent structures were significantly associated with CEAM. Among these features, the maximum SUV within the empyema cavity exhibited the highest diagnostic performance. In particular, the specificity, PPV, and accuracy of the maximum SUV were significantly higher than those of the two other significant
18F-FDG PET/CT findings, that is, the presence of a protruding soft tissue mass and the involvement of adjacent structures. In this study, using the maximum SUV within the empyema cavity as the diagnostic criterion for CEAM, there was only one false-positive case, which histopathology proved to be a chronic inflammation. Therefore, we suggest that
18F-FDG PET/CT be used for the differentiating of CEAM from benign chronic empyema as a complimentary tool to the conventional CT. Although there were no significant differences between the groups of combined significant findings, this may be due to the small number of subjects and high diagnostic performance of the maximum SUV. Furthermore,
18F-FDG PET/CT is a useful tool for the detection of distant metastases, as well as for guiding biopsy.
From a practical point of view, the visual, qualitative PET analysis has the advantages of less effort and time consuming over the quantitative analysis. However, when we performed a visual analysis using the liver activity as a reference, nine additional false-positive cases were found compared to the quantitative analysis, which significantly decreased the diagnostic specificity. In addition, the visual, qualitative analysis has the disadvantage of subjective interpretations, depending on the evaluator. Therefore, quantitative analysis may be mandatory for the differential diagnosis between chronic empyema and CEAM.
Although there have been few clinical reports regarding the usefulness of
18F-FDG PET/CT for diagnosing CEAM (
6789), as well as for evaluating the residual masses after the treatment of PAL (
5), there have been no reports on the diagnostic performance of
18F-FDG PET/CT in diagnosing CEAM in patients with chronic empyema. To the best of our knowledge, this is the first reporting of the high diagnostic performance of
18F-FDG PET/CT.
The clinical features of CEAM in our study were comparable to those reported in previous studies (
11112131415). We found that CEAM developed in elderly patients with a history of tuberculous pleuritis (5/6, 83.3%). The male-to-female ratio in our study was 5.5:1, which may be associated with the male predominance of tuberculosis.
There are several limitations in our study. First, this is a retrospective single-center study, and not all subjects underwent 18F-FDG PET/CT for differentiating CEAM from benign empyema, which may induce selection bias. Second, the study population was of a small size, particularly for patients with CEAM, because it is a rare disease. Therefore, further prospective studies with larger numbers of participants are warranted to investigate the diagnostic value of our suggested 18F-FDG PET/CT criteria for CEAM.
In conclusion, 18F-FDG PET/CT can be useful for differentiating CEAM from benign chronic empyema. Among several useful 18F-FDG PET/CT features, the maximum SUV within the empyema cavity demonstrated the best diagnostic performance for diagnosing CEAM.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download