Abstract
Purpose
Magnetic resonance-guided focused ultrasound (MRgFUS) thalamotomy has become a standard treatment for medically intractable essential tremor (ET). Skull density ratio (SDR) and skull volume in patients with ET are currently considered useful indicators of the successful application of MRgFUS. We compared the clinical outcomes of MRgFUS thalamotomy with SDR above 0.4 and 0.45. We also described patterns of SDR and skull volume in Korean patients with ET who were eligible to be screened for MRgFUS.
Materials and Methods
In screening 318 ET patients, we evaluated patterns of skull density and skull volume according to age and sex. Fifty patients with ET were treated with MRgFUS. We investigated the effects of SDR and skull volume on treatment parameters and the outcomes of ET.
Results
The mean SDR of the 318 ET patients was 0.45±0.11, and that for skull volume was 315.74±40.95 cm3. The male patients had a higher SDR than female patients (p=0.047). Skull volume significantly decreased with aging. SDR and skull volume exhibited a linear negative relationship. Among therapeutic parameters, maximal temperature was positively related to SDR, while sonication number was not related to either SDR or skull volume. Tremor outcome was also not related to SDR or skull volume.
Magnetic resonance-guided focused ultrasound (MRgFUS) is a useful tool for treating patients with intractable essential tremor (ET). Ultrasound is defined as mechanical waves with a frequency higher than human audible sound (conventionally 20000 Hz). It travels through all kinds of material from gases, liquids, to solids. Mechanical waves can be reflected, refracted, or attenuated by the medium.1 The skull is an important barrier for ultrasound traversing into the brain. The skull distorts the ultrasound waves, absorbs energy leading to skull heating, and attenuates the ultrasound beam. Currently, a phased array of up to 1024 ultrasound transducers helps correct skull distortion and concentrate a hot spot.23 Nevertheless, the skull is a wall that introduces ultrasonic power into brain targets. Recently, skull density ratio (SDR) was proposed as one indication for MRgFUS eligibility by our team.4 We suggested an SDR of ≥0.45 and a skull volume ≤330 cm3 as ideal conditions for successful treatment with MRgFUS with a lower energy requirement. Although low SDR (≤0.4) and great skull volume (≥330 cm3) are not contraindications to MRgFUS, higher energy (sonication power, duration, and the number of sonications) may be required to achieve a therapeutic temperature above 54℃ to make a permanent thermal lesion. We applied this criterion for SDR (at least above 0.4) as optimal candidates of a randomized control clinical trial for ET.56 In this paper, we further describe patterns of SDR and skull volume according to age and sex in patients who fulfilled MRgFUS eligibility criteria by screening brain CT. We also analyzed the influence of skull factors (SDR and skull volume) on treatment parameters and outcomes of MRgFUS thalamotomy in ET.
We have collected data since December 2013 for individuals who have undergone screening tests of their skull characteristic with brain CT for MRgFUS suitability. Typical CT scan acquisition parameters were axial, helical acquisition, 1-mm slice thickness with no slice spacing, 512×512 matrix, and bone kernel. Skull volume in the treatment area and SDR were obtained. SDR was defined as the ratio between the mean values in Hounsfield units for marrow and cortical bone, which was calculated as follows: 1) The planned locations of the target and the transducer were marked on CT scans, and the surface of the skull was subdivided into small sections. 2) For each skull subsection, multiple parallel rays were created, and the CT values along each ray were used to create local density profiles. 3) For each profile, the trabecular CT value (local minimum CT value in the profile) and the cortex CT value (average of inner and outer cortices) were computed. 4) The “local” SDR was the average of the ratios between the trabecular and cortex CT values of all of the profile samples.4 All patients provided written informed consent before procedures, and this study received full ethics approval from the Korean Ministry of Food and Drug Safety and local institutional review board (IRB institution: Yonsei University, IRB number 1-2013-0026).
There were 318 patients (231 male and 87 female) who ranged in age from 29 to 86 years old [66.65±9.95, mean±standard deviation (SD)]. We described the patterns of skull density and skull volume according to age and sex in all patients who underwent a screening evaluation. The relationship between SDR and skull volume was obtained with a cutoff value of SDR<0.4, 0.4≤SDR<0.45, and SDR≥0.45.
Among 318 patients who had screening evaluations, 50 patients were treated with MRgFUS for ET. These patients were consecutively enrolled in a randomized controlled trial for treatment with MRgFUS thalamotomy in our institute (ClinicalTrials.gov number: NCT01827904). Eligibility criteria for undergoing MRgFUS thalamotomy were described previously.67 When it comes to SDR, inclusion criteria were 0.45±0.05 or greater. There were 42 males and 8 females. The age range of the patients was from 45 to 80 years old (66.65±9.95). We analyzed if SDR and skull volume had an effect on the treatment parameters (the number of sonications and maximum temperature) and ET outcomes. Estimation of the severity of ET and disability due to ET were previously described.6 In short, tremor severity and task performance were measured as “hand tremor score” of the contralateral hand to the thalamotomy. The scale was assessed by adding the scores of category of the Clinical Rating Scale for Tremor (CRST) Part A of the treated hand (resting, postural, and action tremor; 0–4 scale on each item) and those of the CRST Part B of the treated hand (handwriting, drawing of two Archimedes' spirals and a straight line, and pouring; 0–4 scale on each item). “Postural” and “Action” tremors of CRST Part A were separately encoded to display the motoric aspects of the tremor. “Disability” related to the tremor was measured with the CRST Part C, which comprised seven items with a 0–4 scale on each item. The outcome scores were obtained from the final follow-up visit compared to the baseline scores. Follow-up period was ranged from 1 month to 60 (17.8±19.8) months. The effects on treatment parameters and ET outcomes were divided by SDR≥0.4 and ≥0.45 to see if the cutoff value of SDR≥0.4 had a significant influence on outcomes.
R language version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and T&F program version 2.5 (YooJin BioSoft, Seoul, Korea) were used for all statistical analyses. Continuous variables are expressed as mean±SD, and differences between groups were analyzed using Student's t-test. For categorical variables, data are expressed as numbers and percentages, N (%), and the chi-squared test was used to compare proportions. The Pearson correlation coefficient was computed between SDR and skull volume with and without adjustment for age and sex. Linear regression analysis was performed to analyze the effect of SDR and skull volume on continuous responses with adjustment for age and sex.
The mean SDR of the 318 patients screened was 0.45±0.11 (mean±SD), which ranged from 0.11 to 0.73. The mean skull volume was 315.74±40.95 cm3 and ranged from 224 to 469. SDR was influenced by sex, not by age. Male patients showed a significantly higher value of SDR (p=0.016, Fig. 1). Skull volume, however, was influenced by age (p<0.001), but not by sex. Skull volume decreased with age (Fig. 2). The SDR and skull volume had a negative linear relationship (skull volume=360.977−101.509×SDR). This was maintained when the SDR cutoff value ≥0.4 [skull volume=370.702−119.365×SDR (p=0.001)] or ≥0.45 [skull volume=359.811−99.975×SDR (p=0.031)] (Fig. 3).
When SDR was divided into three groups, 166 patients (52.2%) had an SDR of 0.45 or more, whereas 101 (31.8%) belonged to the group with an SDR of less than 0.4. Age or sex did not show a significant difference among the three groups (p>0.05). Skull volume was significantly smaller in the group with a SDR≥0.45 (Table 1).
Fifty patients with ET were treated with MRgFUS. Mean SDR was 0.51 and ranged from 0.26 to 0.72. Mean skull volume was 305.6 cm3, with a range from 236 to 453. The mean sonication number was 15.12, with a range from 5 to 27. The maximal temperature was 58.7℃ and ranged from 54 to 66 (Table 2). After a mean of 17.8 months of follow up, hand tremor score improved from 12.12±0.51 to 5.88±0.52; action tremor score of the treated hand improved from 2.96±0.1 to 0.86±0.11; postural score improved from 2.54±0.15 to 0.46±0.13; and disability score improved from 12.52±0.52 to 3.64±0.47. The improvements were all statistically significant p<0.001 (Table 3).
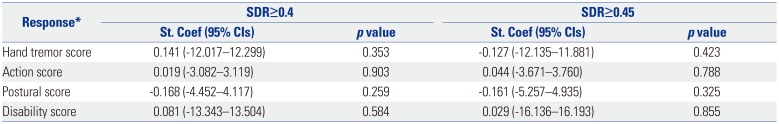
When the relationship between SDR or skull volume and the therapeutic parameters were analyzed, the sonication number was not related with either variable (Table 4), and the maximal temperature was positively related to SDR (p<0.001) (Table 5). Tremor outcome, severity, and disability were not significantly affected by SDR or skull volume (Table 6). When the patients were divided by SDR≥0.4 and SDR≥0.45, the results were similar to the whole group: The maximal temperature was positively related to SDR, but not with skull volume in either group. The sonication number was not related to either variable. The outcomes of tremor were also not influenced by SDR even with SDR≥0.45 (Table 7).
Our study had a larger number of male than female patients, both in the screening group and in the treatment group. However, in the screening group, female patients showed a significantly lower SDR and larger skull volume, compared to male patients. The hormonal effect, which can more often cause osteoporosis in female bone, may explain the reason for lower SDR in female patients.8
After the initial report of SDR4 by our team, we tried to select MRgFUS candidates only above SDR>0.4. This would be also a contributing factor for male dominance in the treated group. We are assuming the SDR in Asian populations is lower than in Western populations. This would be another barrier for finding the optimal MRgFUS thalamotomy candidates for patients with ET.
As previously reported, maximal temperature was affected by SDR.4 The high SDR can easily offer the maximal temperature to make a permanent lesion in the thalamus. However, this did not indicate an improved treatment outcome. The group with the ideal value of SDR≥0.45 and the group with SDR≥0.4 showed a similar positive relationship with maximal temperature and an insignificant influence on outcomes. This finding indicated that SDR only offers an applicability of the procedure; it does not justify the successful outcome unless the lesion is not in the sweet spot of the Vim thalamus. There is a recent report about skull bone marrow injury after this procedure.9 In the study, the authors retrospectively reviewed 30 patients to detect skull bone marrow signal change after MRgFUS after one indexing case of a skull lesion. All patients had SDR≥0.4 and 7 of them had skull lesions, and it was related to maximal energy. To obtain proper tissue temperature, sufficient energy should penetrate the skull properly.
We initially suggested SDR to be above 0.4 to find the best candidates for MRgFUS.4 Additionally, we recently recognized another important skull issue, specifically the incidence beam angle, for the successful treatment with MRgFUS.10 This suggests that SDR alone is not a perfect factor to find optimal candidates for MRgFUS. Most importantly, by improving techniques to prevent the abnormal cavitation signals during MRg- FUS, the procedure became much easier to overcome low SDR-related issues. We recently discovered that patients with low SDR also can be treated with MRgFUS, even if the technique faces some technical difficulties, such as longer treatment time or higher energy. In considering our results as long as we could obtain the therapeutic maximal temperature, SDR alone seems not to be related to MRgFUS treatment outcomes in ET patients.
As we recently demonstrated, various skull factors, such as volume, shape, thickness of marrow, as well as the portion of the cortical bone, also can affect the outcomes of MRgFUS because of the characteristics of the ultrasound.10 Jung, et al.10 demonstrated that if the incidence beam angle of the target is above 25 degrees, the procedure may fail to make an effective thermal lesion. Thus, we need to be aware of the various skull parameters for predicting the creation of successful thermal lesions with MRgFUS.
As time goes by many kinds of procedures for ET shows waning efficacy. In this study, the treatment group included patients with a short follow-up duration. To clarify the factors affecting tremor outcome, more studies are needed.
In conclusion, SDR varied widely and was not affected by age. However, the male patient group had a higher SDR than the female patient group. Skull volume was significantly smaller in cases of higher SDR. The SDR was negatively related to skull volume. SDR and skull volume are still important for eligibility to obtain successful application of MRgFUS from ET patients; however, SDR seems not to affect the clinical outcomes of patients treated with MRgFUS for ET.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI19C0060). Also, the authors thank Eyal Zadicario, Itay Rachmilevitch and other employees of InSightec for their excellent technical assistance.
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Jin Woo Chang.
Data curation: Yong-Sook Park.
Formal analysis: Yong-Sook Park.
Funding acquisition: Jin Woo Chang.
Investigation: Yong-Sook Park.
Methodology: Yong-Sook Park.
Project administration: Jin Woo Chang.
Resources: Kyung Won Chang.
Software: Yong-Sook Park.
Supervision: Jin Woo Chang.
Validation: Jin Woo Chang.
Visualization: Kyung Won Chang.
Writing—original draft: Kyung Won Chang.
Writing—review & editing: Jin Woo Chang.
References
1. Prada F, Kalani MYS, Yagmurlu K, Norat P, Del Bene M, DiMeco F, et al. Applications of focused ultrasound in cerebrovascular diseases and brain tumors. Neurotherapeutics. 2019; 16:67–87. PMID: 30406382.

2. Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002; 8(Suppl 1):S100–S112. PMID: 12075696.
3. Connor CW, Hynynen K. Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery. IEEE Trans Biomed Eng. 2004; 51:1693–1706. PMID: 15490817.

4. Chang WS, Jung HH, Zadicario E, Rachmilevitch I, Tlusty T, Vitek S, et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg. 2016; 124:411–416. PMID: 26361280.

5. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016; 375:730–739. PMID: 27557301.

6. Chang JW, Park CK, Lipsman N, Schwartz ML, Ghanouni P, Henderson JM, et al. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: results at the 2-year follow-up. Ann Neurol. 2018; 83:107–114. PMID: 29265546.

7. Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013; 369:640–648. PMID: 23944301.

9. Schwartz ML, Yeung R, Huang Y, Lipsman N, Krishna V, Jain JD, et al. Skull bone marrow injury caused by MR-guided focused ultrasound for cerebral functional procedures. J Neurosurg. 2018; 4. 01. [Epub]. DOI: 10.3171/2017.11.JNS17968.

10. Jung NY, Rachmilevitch I, Sibiger O, Amar T, Zadicario E, Chang JW. Factors related to successful energy transmission of focused ultrasound through a skull: a study in human cadavers and its comparison with clinical experiences. J Korean Neurosurg Soc. 2019; 5. 30. [Epub]. DOI: 10.3340/jkns.2018.0226.
Fig. 1
Relationship for skull density ratio (SDR) in all screening patients with essential tremor. (A) Linear regression line between age and SDR (p>0.05). (B) Comparison of mean differences in SDR between male and female groups. Male patients show significantly higher SDR (p<0.05).
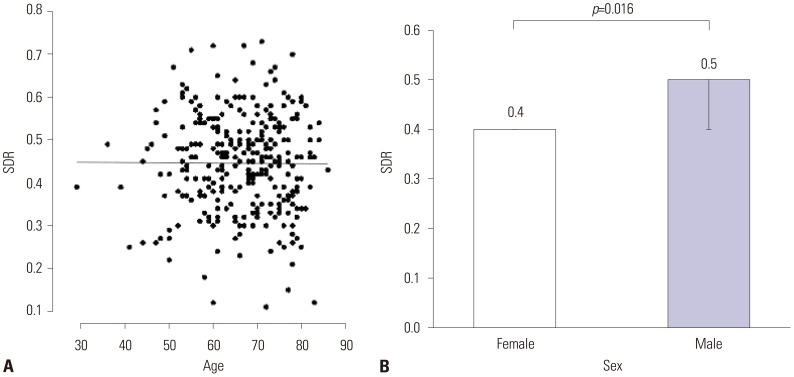
Fig. 2
Relationship for skull volume in all screening patients with essential tremor (n=318). (A) Linear regression line between skull volume and age (p<0.001). (B) Comparison of mean differences in skull volume in the male and female patients. No difference is present.
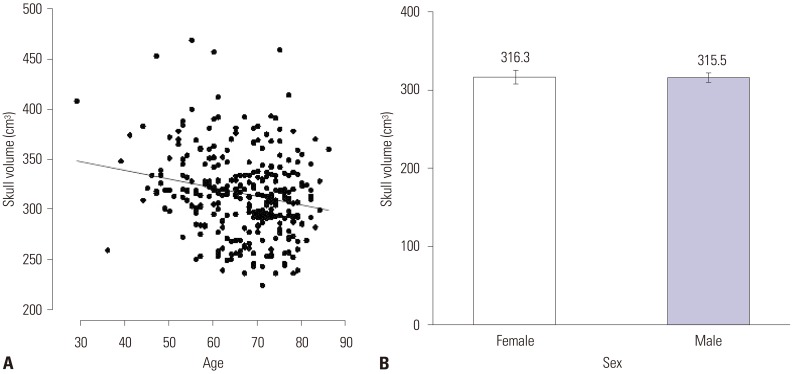
Fig. 3
The relationship between skull density ratio (SDR) and skull volume. The SDR and skull volume exhibited a negative linear relationship. (A) Skull volume=360.977−101.509×SDR for correlation of SDR and skull volume in SDR≥0.4. (B) Skull volume=370.702−119.365×SDR (p=0.001) for SDR≥0.45. (C) Skull volume=359.811−99.975×SDR (p=0.031). They all showed a significant relationship.
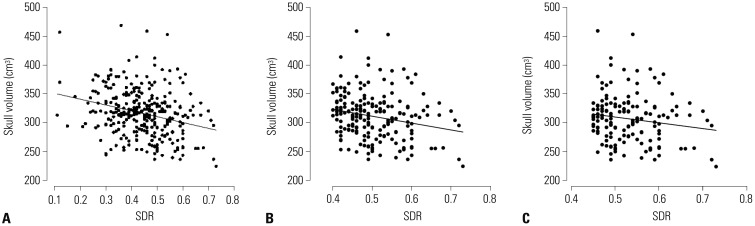
Table 1
Baseline Characteristics of the Screening Patients
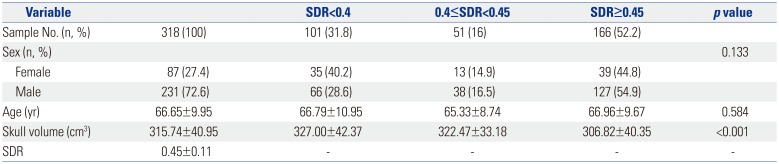
Table 2
Characteristics of Magnetic Resonance-Guided Focused Ultrasound Treated Patients and Therapeutic Parameters
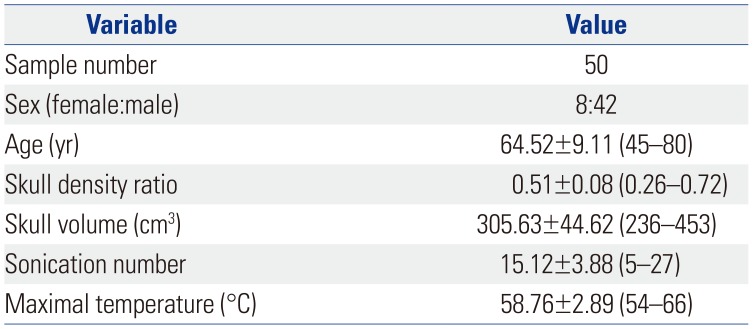
Table 3
Tremor Outcome Scores and Comparison of Mean Differences between Baseline and Follow Up
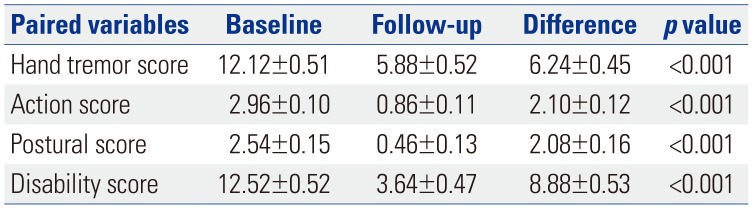
Table 4
Results of Univariable Linear Regression Analysis Using Sonication as a Response
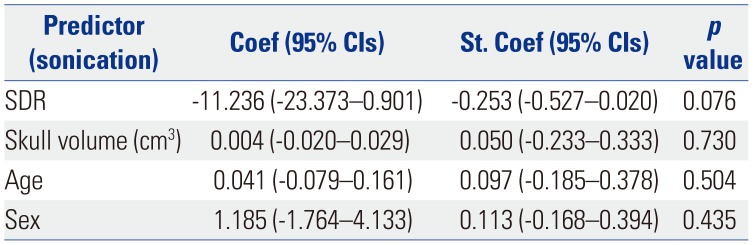
Table 5
Results of Univariable Linear Regression Analysis Using Maximal Temperature as Responses
Table 6
Relation of Tremor Outcomes according to Skull Density Ratio and Skull Volume Adjusted for Age and Sex
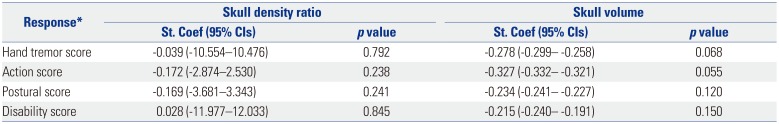
Table 7
Relation of Tremor Outcome in SDR≥0.4 and in SDR≥0.45 Adjusted for Age and Sex




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download