1. Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg. 1993; 51:742–747. discussion 748–9. PMID:
8509912.

2. Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010; 14:287–297. PMID:
20189852.

3. Liu SY, Riley RW. Continuing the original Stanford sleep surgery protocol from upper airway reconstruction to upper airway stimulation: our first successful case. J Oral Maxillofac Surg. 2017; 75:1514–1518. PMID:
28294946.

4. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009; 5:263–276. PMID:
19960649.
5. Pirklbauer K, Russmueller G, Stiebellehner L, Nell C, Sinko K, Millesi G, et al. Maxillomandibular advancement for treatment of obstructive sleep apnea syndrome: a systematic review. J Oral Maxillofac Surg. 2011; 69:e165–e176. PMID:
21605790.

6. Riley RW, Powell NB, Guilleminault C. Maxillofacial surgery and nasal CPAP. A comparison of treatment for obstructive sleep apnea syndrome. Chest. 1990; 98:1421–1425. PMID:
2245683.
7. Prinsell JR. Maxillomandibular advancement surgery for obstructive sleep apnea syndrome. J Am Dent Assoc. 2002; 133:1489–1497. quiz 1539–40. PMID:
12462692.

8. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188:996–1004. PMID:
23721582.

9. Kyung SH, Park YC, Pae EK. Obstructive sleep apnea patients with the oral appliance experience pharyngeal size and shape changes in three dimensions. Angle Orthod. 2005; 75:15–22. PMID:
15747810.
10. Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015; 11:861–868. PMID:
25845897.

11. Hoekema A, de Lange J, Stegenga B, de Bont LG. Oral appliances and maxillomandibular advancement surgery: an alternative treatment protocol for the obstructive sleep apnea-hypopnea syndrome. J Oral Maxillofac Surg. 2006; 64:886–891. PMID:
16713801.

12. Schendel SA, Wolford LM, Epker BN. Mandibular deficiency syndrome. III. Surgical advancement of the deficient mandible in growing children: treatment results in twelve patients. Oral Surg Oral Med Oral Pathol. 1978; 45:364–377. PMID:
273187.
13. Mojdehi M, Buschang PH, English JD, Wolford LM. Postsurgical growth changes in the mandible of adolescents with vertical maxillary excess growth pattern. Am J Orthod Dentofacial Orthop. 2001; 119:106–116. PMID:
11174555.

14. Ahn HW, Lee BS, Kim SW, Kim SJ. Stability of modified maxillomandibular advancement surgery in a patient with preadolescent refractory obstructive sleep apnea. J Oral Maxillofac Surg. 2015; 73:1827–1841. PMID:
25865720.

15. Han S, Choi YJ, Chung CJ, Kim JY, Kim KH. Long-term pharyngeal airway changes after bionator treatment in adolescents with skeletal Class II malocclusions. Korean J Orthod. 2014; 44:13–19. PMID:
24511511.

16. Doff MH, Jansma J, Schepers RH, Hoekema A. Maxillomandibular advancement surgery as alternative to continuous positive airway pressure in morbidly severe obstructive sleep apnea: a case report. Cranio. 2013; 31:246–251. PMID:
24308097.

17. McNamara JA Jr. Dentofacial adaptations in adult patients following functional regulator therapy. Am J Orthod. 1984; 85:57–71. PMID:
6581727.

18. Ruf S, Pancherz H. Dentoskeletal effects and facial profile changes in young adults treated with the Herbst appliance. Angle Orthod. 1999; 69:239–246. PMID:
10371429.
19. Liao YF, Chiu YT, Lin CH, Chen YA, Chen NH, Chen YR. Modified maxillomandibular advancement for obstructive sleep apnoea: towards a better outcome for Asians. Int J Oral Maxillofac Surg. 2015; 44:189–194. PMID:
25305697.

20. Liou EJ, Chen PH, Wang YC, Yu CC, Huang CS, Chen YR. Surgery-first accelerated orthognathic surgery: postoperative rapid orthodontic tooth movement. J Oral Maxillofac Surg. 2011; 69:781–785. PMID:
21353934.

21. Wang Q, Jia P, Anderson NK, Wang L, Lin J. Changes of pharyngeal airway size and hyoid bone position following orthodontic treatment of Class I bimaxillary protrusion. Angle Orthod. 2012; 82:115–121. PMID:
21793712.

22. Hu Z, Yin X, Liao J, Zhou C, Yang Z, Zou S. The effect of teeth extraction for orthodontic treatment on the upper airway: a systematic review. Sleep Breath. 2015; 19:441–451. PMID:
25628011.

23. Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007; 30:461–467. PMID:
17520790.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



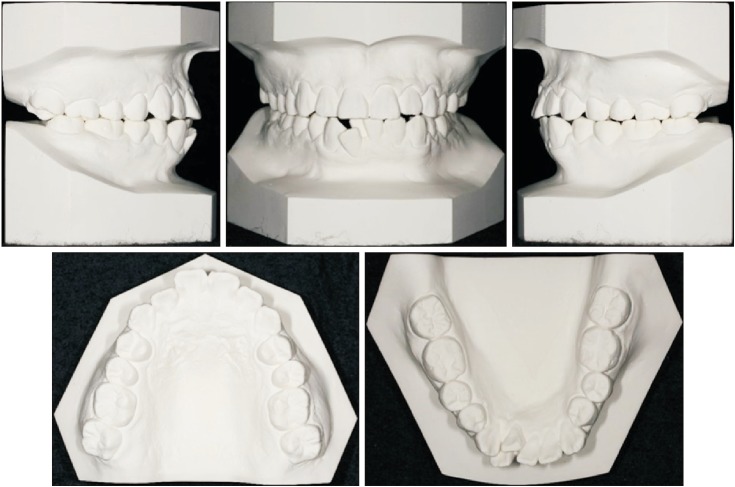
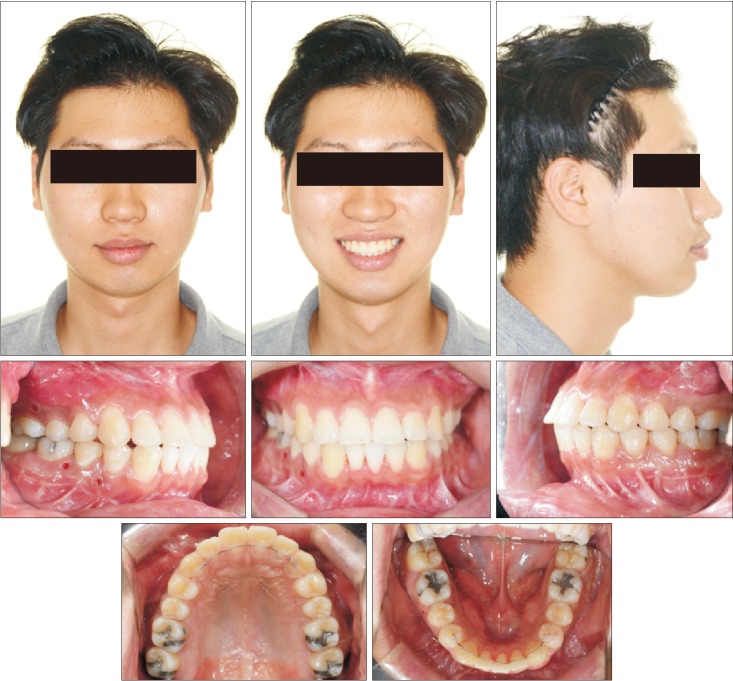
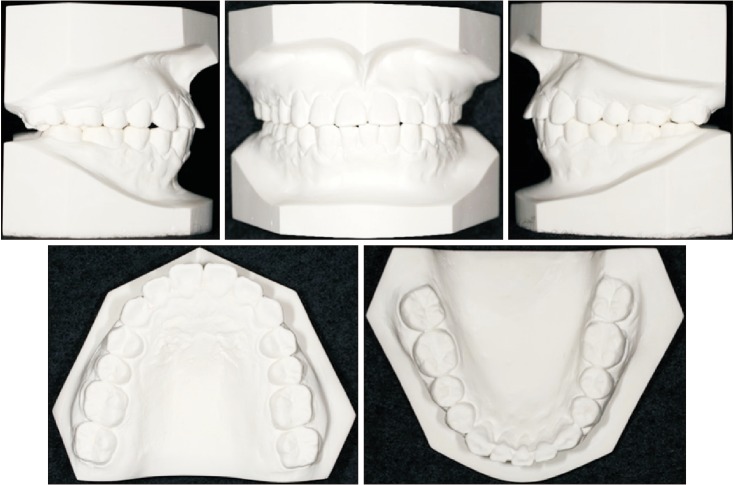
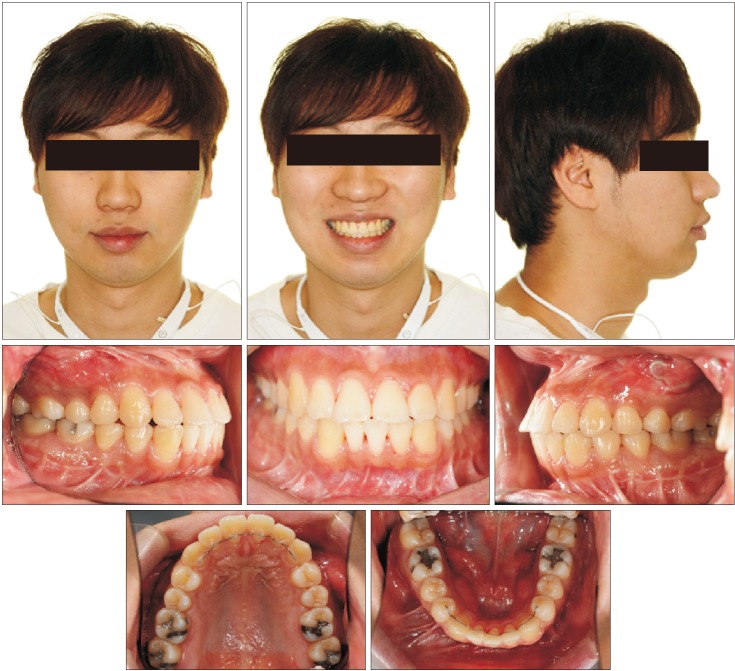
 XML Download
XML Download