Abstract
Objective
Methods
Results
Figures and Tables
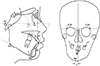 | Figure 1Cephalometric measurements.1, Sella-Nasion to A Point angle (SNA); 2, sella-nasion to B point angle (SNB); 3, A point-Nasion to B point angle (ANB); 4, upper incisor to Frankfort plane angle (U1-FH); 5, lower incisor to mandibular plane angle (IMPA); 6, menton deviation-the distance from the perpendicular bisector line of both frontozygomatic points (FZPs) to the menton.
|
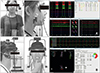 | Figure 2A, Electromyographic recording device (Bio-EMG III™; BioResearch, Inc., Milwaukee, WI, USA) and the data for clenching obtained from one subject. B, Jaw movement tracking device (JT-3D™; BioResearch, Inc.) and the sequence for right-sided gum chewing obtained from one subject. |
 | Figure 3Schematic drawing of the jaw movement. A, Two cycles of the three-dimensional mandibular incisor-point chewing sequences. The three graphs indicate the tracing in the vertical (upper), anteroposterior (middle), and lateral (lower) axes. The dotted vertical line indicates the “turning point” between opening and closing of the mouth. B, Average chewing pattern in the frontal and horizontal view. Opening and closing angles in the frontal or horizontal view were defined as the angles between each plane and the tangent line of the average chewing trajectory. |
 | Figure 4The mean paths of the average chewing pattern during unilateral gum chewing at T0 (blue) and T1 (red) in the frontal and horizontal views.T0, Initial; T1, 7–8 months after orthognathic surgery.
|
Table 2
Inter- and intragroup comparisons of cephalometric measurements

Values are presented as mean ± standard deviation.
Expermimental group, Class III facial asymmetry; Control group, Class III without facial asymmetry; T0, initial; T1, 7–8 months after orthognathic surgery.
Paired t-test was performed: *p < 0.05, **p < 0.01, ***p < 0.001; Independent t-test was performed: †p < 0.001.
See Figure 1 for definitions of each landmark or measurement.
Table 3
Inter- and intragroup comparison of electromyography (EMG) potentials

Values are presented as mean ± standard deviation.
In the control group, the measurements on the left and the right sides were pooled together for statistical analysis because the differences between the two sides were not significant.
Expermimental group, Class III facial asymmetry; Control group, Class III without facial asymmetry; T0, initial; T1, 7–8 months after orthognathic surgery; TA_D, EMG activity of the anterior temporalis muscle (TA) on the deviated side; TA_ND, EMG activity of the TA on the non-deviated side; MM_D, EMG activity of the superficial masseter muscle (MM) on the deviated side; MM_ND, EMG activity of the superficial MM on the non-deviated side; TA_Rt, EMG activity of the TA on the right side; TA_Lt, EMG activity of the TA on the left side; MM_Rt, EMG activity of the superficial MM on the right side; MM_Lt, EMG activity of the superficial MM on the left side; As, asymmetry index; Ac, activity index.
Paired t-test was performed: *p < 0.05; **p < 0.01; †Independent t-test was performed.
Table 4
Inter- and intragroup comparison of jaw movement variables in the range of motion (mm)

Values are presented as mean ± standard deviation.
Expermimental group, Class III facial asymmetry; Control group, Class III without facial asymmetry; T0, initial; T1, 7–8 months after orthognathic surgery.
Paired t-test was performed: *p < 0.05, **p < 0.01; Independent t-test was performed: †p < 0.05, ‡p < 0.01.
Table 5
Inter- and intragroup comparison of jaw movement variables of unilateral average chewing pattern (ACP)

Values are presented as mean ± standard deviation.
Expermimental group, Class III facial asymmetry; Control group, Class III without facial asymmetry; T0, initial; T1, 7–8 months after orthognathic surgery; D, chewing in the deviated side; ND, chewing in the non-deviated side; C, unilateral chewing in the control group; OT, opening time; CT, closing time; OCT, occlusal time; CYT, cycle time; AP, anteroposterior; Mx, maximum; OP, opening; CL, closing; Av, average; F, frontal; H, horizontal.
Paired t-test was performed: *p < 0.05; Independent t-test was performed: †p < 0.05, ‡p < 0.01, §p < 0.001.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download