MATERIALS AND METHODS
1. Patients
Upon receiving Institutional Review Board approval (IRB Number 4-2018-1141), we retrospectively reviewed patients who had been histologically or clinically diagnosed with recurrent EOC at our center from 2010 to 2017. Informed consent was waived because of the retrospective nature of the study. All patients were initially treated with optimal cytoreductive surgery, followed by adjuvant platinum-based chemotherapy according to their pathologic stage. The inclusion criterion was treatment with tumor-directed IFRT either at the time of diagnosis of recurrent ovarian cancer or after salvage therapies, including chemotherapy and/or cytoreductive surgery. The exclusion criteria included a lack of initial optimal cytoreductive surgery, the presence of brain metastasis, or a palliative RT dose <45 Gy (equivalent dose in 2 Gy/fraction [EQD2]). We also excluded 31 patients from a prospective cohort enrolled in our pervious phase II trial [
6]. After applying these exclusion criteria, 61 patients were ultimately analyzed.
2. IFRT
Patients were categorized into 3 groups according to the sequence of chemotherapy and IFRT. Based on Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST 1.0) [
7], consolidative IFRT was only considered for patients presenting a metabolic complete response. Other sequences include salvage IFRT upon stable disease or progressive disease based on either Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) [
8] or PERCIST (version 1.0) after chemotherapy. Patients who received the initial IFRT without chemotherapy were categorized under salvage IFRT without chemotherapy.
During each IFRT treatment, all identified lesions were treated with intensity-modulated, stereotactic body, or 3-dimensional conformal RT. The consistency of target volume was confirmed by a single gynecologic radiation oncologist (YBK). All patients underwent computed tomography (CT) simulations using immobilization devices depending on the lesion site. After CT simulation, the gross tumor volume (GTV) was contoured on the CT scan to include all visible lesions or the tumor bed defined following chemotherapy. The clinical target volume (CTV) was defined as an expansion of the GTV of up to 10–15 mm, as well as adjacent high-risk regions (i.e. initial tumor before chemotherapy) at the discretion of the treating physician. Therefore, 2 planning target volumes (PTV) could be outlined. Initial PTV is CTV including GTV with a set-up margin; boost PTV is for GTV alone with a set-up margin. Generally, we prescribed a total 37.5 Gy in 15 fractions for initial PTV and a total of 45 Gy in 15 fractions to boost PTV using a simultaneous integrated boost. We alternatively prescribed a total of 44 Gy in 20 fractions for initial PTV and a 50 Gy in 20 fractions to boost PTV when radiosensitive organs, such as the small bowel or duodenum, were close to PTV. For stereotactic body radiation therapy, a total of 30 Gy in 5 fractions or 32 Gy in 4 fractions were prescribed. Given the variability in the treated sites, no uniform dose constraints were placed for organs-at-risk. To correlate different irradiation doses with the clinical results, the EQD2 was calculated using an assumed α/β-ratio of 10 for the tumor.
3. Follow-up
All patients were followed up until death or the time of analysis. At 3 months after planned RT, most patients underwent a history-taking and physical examination, standard imaging evaluations, and laboratory testing, including a measure of the carbohydrate antigen-125 (CA-125) level. The responses and progression of all tumors were evaluated using the revised RECIST (version 1.1) [
8]. Local failure was defined as tumor progression at the treated site. In patients in whom local failures were determined from serial assessments, each event was documented from the first radiographic appearance of tumor progression. After progressive disease following RT was confirmed, multidisciplinary team discussion based on current evidence and consensus settled issues regarding subsequent salvage treatments such as chemotherapy, supportive care, or re-irradiation. Treatment-related toxicity events were graded at the time of follow-up according to the Common Terminology Criteria for Adverse Events (version 4.03) [
9].
4. Statistical analysis
The in-field control rate (IFCR) was calculated from the first date of IFRT to the date of subsequent failure within the IFRT field (i.e., in-field). Progression-free survival (PFS) was defined as the time interval from IFRT to subsequent progression (either in-field or out-field) or death without disease, whichever occurred first. Overall survival (OS) was calculated in months from the first date of IFRT to the date of death, or to the last follow-up visit for patients who remained alive. In patients who did not receive concomitant chemotherapy, the chemotherapy-free interval (CFI) was calculated from the date of first IFRT to the first date of administration of the next chemotherapy course or the last follow-up.
The Kaplan-Meier method was used to estimate the OS, PFS, IFCR, and CFI. A Cox regression model was used for the multivariable analysis of factors affecting OS, PFS, IFCR, and CFI. In all analyses, a 2-sided p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS statistical software, ver. 23.0 (IBM, Inc., Armonk, NY, USA).
RESULTS
The 61 consecutive patients with 90 treatments who were included in this study had a median age of 54 (interquartile range [IQR], 49–61) years. The most common histology was serous carcinoma and was present in 49 (80.3%) patients. Patients underwent IFRT after a median of 2.0 (IQR, 2.0–2.4) months after the last chemotherapy course. The median CA-125 levels at the time of IFRT and at 3 months after IFRT were 30.0 (IQR, 10.0–87.1) and 18.1 (IQR, 5.6–123.3) U/mL, respectively. More than half of patients received 2 or 3 courses of chemotherapy before IFRT. Details of the patients' characteristics are presented in
Table 1.
Table 1
Patient and treatment characteristics

|
Characteristics |
Per patient (n=61) |
Per treatment (n=90) |
|
Age (yr) |
54.0 (48.5–61.0) |
53.5 (48.0–60.0) |
|
ECOG PS |
|
|
|
ECOG 0–1 |
53 (86.9) |
77 (85.4) |
|
ECOG 2–3 |
8 (13.1) |
13 (14.4) |
|
Pathology |
|
|
|
Serous |
49 (80.3) |
75 (83.3) |
|
Endometrioid |
5 (8.2) |
7 (7.8) |
|
Clear cell |
4 (6.6) |
5 (5.6) |
|
Other |
3 (4.9) |
3 (3.3) |
|
BRCA |
|
|
|
Negative |
17 (27.9) |
24 (26.7) |
|
BRCA1 |
10 (16.4) |
15 (16.7) |
|
BRCA2 |
4 (6.6) |
5 (6.7) |
|
BRCA1/2 |
2 (3.3) |
6 (5.6) |
|
Unknown |
28 (45.9) |
40 (44.4) |
|
Platinum sensitivity |
|
|
|
Platinum sensitive (mo)*
|
10.5 (5.0–23.4) |
11.3 (5.7–22.3) |
|
Sensitive (≥6 mo) |
26 (42.6) |
37 (41.4) |
|
Resistant (<6 mo) |
35 (57.4) |
53 (58.9) |
|
Carcinomatosis before IFRT |
|
|
|
Absent |
32 (52.5) |
41 (45.6) |
|
Present |
29 (47.5) |
49 (54.4) |
|
CA-125 before IFRT |
35.6 (9.9–86.4) |
30.0 (10.0–87.1) |
|
CA-125 after IFRT |
17.6 (7.8–114.0) |
18.1 (5.6–123.3) |
|
Courses of chemotherapy before IFRT |
|
|
|
1 |
12 (19.7) |
12 (13.3) |
|
2 |
29 (47.5) |
43 (47.8) |
|
3 |
14 (23.0) |
21 (23.3) |
|
4–5 |
6 (9.8) |
14 (15.5) |
|
CFI before IFRT (mo)†
|
2.0 (1.4–5.3) |
2.3 (1.5–10.2) |

One-third of the treatment cases (30 cases, 33.3%) met the category of consolidation IFRT after a metabolic complete response to chemotherapy, 27 cases (30.0%) involved salvage IFRT despite a variable or poor response to chemotherapy, and 33 cases (36.7%) were categorized into initial salvage IFRT without chemotherapy. Ninety treatments were administered to 61 patients at a median prescription dose of EQD2 50.8 (IQR, 48.8–55.9) Gy and a median dose per fraction of 2.2 (IQR, 2.0–2.5) Gy. Individual doses were based on the target volume. The prescribed dose significantly differed among sequences of IFRT (p<0.003). For 30 cases with consolidative IFRT, a median prescription dose was EQD2 47.5 (IQR, 44.0–54.0) Gy and a median dose per fraction of 2.1 (IQR, 2.0–3.0) Gy. A median EQD2 54 (IQR, 50.0–56.2) Gy was prescribed in the salvage IFRT following chemotherapy, and a median EQD2 50.0 (IQR, 45–54 Gy) was prescribed in the salvage IFRT without chemotherapy. Most treatments were administered to extra-abdominal sites with regional area (36 cases, 40.0%) following treatments to nodal areas such as pelvic, paraaortic, or inguinal lymph nodes (25 cases, 27.8%). A total of 172 sites were treated, of which the paraaortic and mediastinal lymph nodes were the most frequent. Further treatment details are presented in
Table 2 and
Supplementary Table 1.
Table 2
Treatment details of IFRT
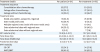
|
Treatment details |
Per patient (n=61) |
Per treatment (n=90) |
|
Treatment sequence |
|
|
|
Consolidation after chemotherapy |
23 (37.7) |
30 (33.3) |
|
Salvage after chemotherapy |
24 (39.3) |
27 (30.0) |
|
Salvage without chemotherapy |
14 (23.0) |
33 (36.7) |
|
Treatment sites |
|
|
|
Nodal area (pelvic, paraaortic, inguinal) |
15 (24.6) |
25 (27.8) |
|
Extra-nodal pelvic area |
10 (26.4) |
12 (13.3) |
|
Multiple regional area (nodal with extra-nodal area) |
7 (11.5) |
10 (11.1) |
|
Extra-abdominal sites with regional area |
24 (39.3) |
36 (40.0) |
|
Extra-abdominal sites without regional area |
5 (8.2) |
7 (7.8) |
|
GTV volume (mL) |
12.8 (4.4–31.1) |
12.7 (4.3–29.6) |
|
Total dose (Gy) |
50.4 (48.4–55.0) |
50.1 (45.0–55.0) |
|
Total dose (EQD2) (Gy) |
52.1 (49.5–55.9) |
50.8 (48.8–55.9) |
|
Fractional dose (Gy) |
2.0 (2.0–2.4) |
2.2 (2.0–2.5) |
|
IFRT modality |
|
|
|
3D CRT |
33 (54.1) |
34 (37.8) |
|
IMRT/SBRT |
28 (45.9) |
56 (62.2) |

During a median follow-up of 19.0 (IQR, 8.6–34.9) months, the 1- and 2-year IFCRs and median in-field control duration were 66.9%, 42.7%, and 18.4 months, respectively. Forty-eight cases (53.3%) experienced in-field failures at a median interval of 9.6 (IQR, 5.1–18.1) months after IFRT. Early in-field failures within 12 months (n=44) have shown distinct pre-treatment features compared to that of others. The median CA-125 level at the time of IFRT of early failures was 52.8 (IQR, 15.6–113.1) compared to 19.5 (IQR, 7.8–65.4) U/mL of other cases (p=0.012). There are certain circumstances for early recurrences; treatment-resistant tumors such as 3 or more cycles of pre-IFRT chemotherapy (n=4), previous IFRT (n=2), platinum-resistant tumors (n=9), high CA-125 level at the time of IFRT (n=26), or prescription dose less than EQD2 50 Gy (n=3). The median follow-up period for patients with disease control status after IFRT was 14.8 (IQR, 7.2–32.8) months. In the multivariate analysis, a higher prescribed dose (EQD2 ≥50 Gy) was associated with better in-field control (p=0.002) (
Supplementary Table 2).
Following IFRT, 53 cases (58.9%) received salvage chemotherapy after progression at a median CFI of 10.5 (95% confidence interval [CI]=7.3–13.7) months (
Fig. 1). Further analysis identified platinum sensitivity (p=0.020) and the CA-125 level at the time of IFRT (p<0.001) as significant prognostic factors for the re-administration of salvage chemotherapy after IFRT (
Supplementary Fig. 1). There was no statistical difference in CFI according to GTV volume, treatment sequence, pre-treatment carcinomatosis, and CFI before IFRT (
Table 3). As shown in
Fig. 1, the 2-year PFS and OS rates of the entire cohort were 24.2% and 78.9%, respectively.
 | Fig. 1
CFR, PFS, and OS of the entire cohort.
CFR, chemotherapy-free rate; OS, overall survival; PFS, progression-free survival.

|
Table 3
Prognostic factors of interval of chemotherapy administration after IFRT

|
Prognostic factors |
2yr CFI (%) |
Univariate analysis |
Multivariate analysis |
|
HR |
95% CI |
p |
HR |
95% CI |
p |
|
Age at IFRT (yr) |
33.9 |
1.04 |
0.61–1.78 |
0.889 |
- |
- |
- |
|
(≥median vs. <median) |
37.1 |
|
Pathology |
31.8 |
1.43 |
0.67–3.06 |
0.352 |
- |
- |
- |
|
(serous vs. others) |
46.7 |
|
Platinum sensitivity |
24.5 |
2.71 |
1.46–5.02 |
0.002 |
2.13 |
1.13–4.01 |
0.020 |
|
(resistant vs. sensitive) |
52.3 |
|
CA-125 before IFRT (U/mL) |
12.4 |
4.59 |
2.54–8.28 |
<0.001 |
4.04 |
2.21–7.38 |
<0.001 |
|
(≥35 vs. <35) |
58.3 |
|
Total dose (EQD2) (Gy) |
20.0 |
1.59 |
0.89–2.85 |
0.115 |
- |
- |
- |
|
(<50 vs. ≥50) |
41.4 |
|
GTV (mL) |
24.1 |
1.57 |
0.91–2.71 |
0.108 |
- |
- |
- |
|
(≥median vs.<median) |
45.6 |
|
Treatment sequence |
35.1 |
1.10 |
0.60–2.00 |
0.763 |
- |
- |
- |
|
(salvage vs. consolidation) |
37.7 |
|
Carcinomatosis before IFRT |
26.4 |
1.44 |
0.83–2.51 |
0.192 |
- |
- |
- |
|
(present vs. absent) |
47.9 |
|
CFI before IFRT (mo) |
39.6 |
1.02 |
0.60–1.76 |
0.929 |
- |
- |
- |
|
(<median vs. ≥median) |
31.5 |

A subgroup analysis stratified by the CA-125 level (35 U/mL) at the time of IFRT and platinum sensitivity revealed disparities in CFI (
Fig. 2). Patients with a normal CA-125 level at the time of IFRT and platinum-sensitive tumors (n=25) had the longest median CFI of 12.6 (IQR, 7.0–25.7) month, with 1- and 2-year CFI rates of 86.9% and 66.8%, respectively. They did not reach median OS with 2-year OS rate of 82.9%. By contrast, patients with an elevated CA-125 level and platinum-resistant tumors (n=31) had the shortest CFI, with a median duration of 4.7 (IQR, 3.0–7.1) months. Median OS in this group was reached at 23.3 months and 2-year OS was 47.2%. Patients with a normal CA-125 level and platinum-resistant tumors (n=22) exhibited a relatively longer median CFI after IFRT when compared to those with an abnormal CA-125 level and platinum-sensitive tumors (n=12) (median CFI, 17 [IQR, 5.4–26.1] vs. 9.8 [IQR, 2.6–10.8] months; 2-year CFI rate, 47.9% vs. 20.8%; p<0.001).
 | Fig. 2
Chemotherapy-free rates prior to involved-field radiation therapy and platinum sensitivity, stratified by the CA-125 level.
CA-125, carbohydrate antigen-125.

|
We further discriminated long-term chemotherapy free group, long-term group (28 cases, 31.1%), from the short-term chemotherapy free group, short-term group (62 cases, 68.9%), based on a CFI of >12 months after IFRT (
Supplementary Table 3). Each group had a median CFI of 27.4 (IQR, 19.4–36.6) and 5.0 (IQR, 2.9–7.4) months, respectively. In 13 of the 28 cases (46.4%) involving the long-term group, IFRT was limited to a single site; otherwise, multiple sites were treated, including 2 sites in 7 cases, 3 sites in 5 cases, and 4 sites in 3 cases. Notably, the proportion of platinum-sensitive tumors was much higher in the long-term group. Although the rate of pre-existing peritoneal carcinomatosis did not differ between the groups, significant differences in the CA-125 level before RT and the change in CA-125 (ratio of CA-125 from before to after IFRT) were observed between the long-term and short-term groups. These groups also differed significantly in terms of OS, with 2-year rates of 92.7% and 67.8%, respectively (p=0.003,
Fig. 3).
 | Fig. 3
OS stratified by the chemotherapy free after involved-field radiation therapy.
OS, overall survival; CFI, chemotherapy-free interval.

|
In an analysis of the subgroup of cases with an elevated CA-125 value (≥35 U/mL) before IFRT, the long-term group (5 cases) and short-term group (38 cases) differed significantly in terms of the change in CA-125 levels (0.34 vs. 1.69, p<0.001). By contrast, in the subgroup of cases with a normal CA-125 value before IFRT, the change in CA-125 levels did not differ statistically between 2 groups (0.98 vs. 3.15, p=0.176).
1. Toxicity
IFRT was well-tolerated and usually did not induce major acute treatment-related toxicities. The acute and late toxicities reported in this study are shown in
Supplementary Table 4. No grade 3 toxicity events were recorded during or shortly after IFRT. Most patients (39 patients, 43.3%) experienced grade 1 or 2 nausea. Furthermore, no grade 3 or more late toxicity events were reported.
DISCUSSION
In this single-institutional cohort study, we retrospectively reviewed 90 cases of IFRT administered to 61 patients with recurrent EOC. Notably, patients in a third of these cases remained free from additional chemotherapy for more than 12 months after IFRT. Furthermore, we identified both platinum sensitivity and the CA-125 level as independent factors associated with chemotherapy re-administration. Specifically, patients with both platinum-sensitive tumors and a CA-125 level within the normal range before IFRT had the longest CFI after IFRT. The long-term chemotherapy free group that achieved a CFI of >12 months had the following distinct tumor and treatment characteristics: platinum sensitivity and a higher EQD2 dose (≥50 Gy) delivered with curative intent, irrespective of the GTV. A moderate decrease in the CA-125 level after IFRT was also found to correlate with the long-term CFI outcomes of patients with initially abnormal CA-125 levels. Our experience suggests that the appropriate determination of patients with recurrent EOC who would most benefit from IFRT could yield the achievement of long-term control without requiring further chemotherapy.
Several previous studies have explored the ability of IFRT to provide favorable local control outcomes in patients with recurrent ovarian cancer [
410111213141516]. The largest single-center retrospective experience of IFRT, conducted by Brown et al. [
4], reported that 35% of patients had no evidence of disease at 28 months after IFRT, as well as a 5-year local control rate of 71%. Brown and colleagues [
4] also demonstrated a notably longer treatment break immediately after IFRT than immediately prior to IFRT, although these chemotherapy breaks decreased gradually with each additional chemotherapy course.
Brown and colleagues noted that in their cohort of 102 patients, most treatment sites were located in the pelvis (54%) and abdomen (44%) [
4]. Recently, Lazzari et al. [
10] studied 109 cases of stereotactic body radiation therapy in 82 patients with oligometastatic ovarian cancer who received a total dose of 24 Gy in 3 fractions to the following treatment sites: abdomen/pelvis (79%), thorax (17%), and head and neck (4%). The authors demonstrated that 47.7% (52 cases) remained chemotherapy-free for a median duration of 7.3 months. These findings are consistent with our observation that IFRT is associated with good long-term control in selected patients; specifically, we observed a median CFI of 10.5 months for the entire cohort vs. 24.7 months for the long-term group. However, the distribution of treatment sites in our cohort was unique, as the cases were roughly equally divided among the pelvis (31.9%), abdomen (36.0%), and other sites (32.1%), including the mediastinum, supraclavicular and axillary lymph nodes. We also observed the achievements of disease control status after IFRT even after including up to 5 treatment sites.
Measurement of the serum CA-125 level is a widely available means of determining the tumor burden and evaluating treatment responses in patients with EOC [
17]. Several studies have already confirmed the CA-125 level as a predictor of the tumor burden after cytoreductive surgery. For example, Suidan et al. [
18] defined 3 clinical and 8 radiologic criteria for the prediction of suboptimal surgery and reported that a CA-125 level >600 U/mL was significantly associated with residual disease after debulking surgery. Consistent with those earlier findings, we identified an elevated CA-125 level as a significant independent factor affecting treatment outcomes, suggesting that a normalized CA-125 level before IFRT reflects a microscopic residual tumor burden after chemotherapy. In this context, gross tumor-directed IFRT could have a considerable effect on treatment outcomes. However, we observed 5 cases with achievements of long-term chemotherapy delay among patients with higher CA-125 levels. Further analysis revealed that those cases exhibited significant changes in CA-125 levels from before to after IFRT. Further investigation is needed to determine the mechanisms underlying the observed discrepancies in the changes in CA-125 levels after IFRT.
We also demonstrated that platinum sensitivity is an important factor affecting CFI after IFRT, consistent with the findings of previous series [
419]. Although patients with platinum-resistant tumors had poor outcomes after IFRT, the 22 patients in this category who had normal CA-125 levels before IFRT might still have benefitted from IFRT in terms of a longer CFI. Other clinicopathological factors, such as age, pathology, peritoneal carcinomatosis, and extent of the GTV, did not appear to affect the applications of IFRT.
Our results provide insights into potential clinical situations wherein IFRT might be useful for the treatment of recurrent EOC, including consolidation in cases with favorable responses after salvage chemotherapy, salvage for cases involving variable post-chemotherapy responses of recurrent lesions, and lesions that recurred outside the extent of debulking surgery or in patients who refused further chemotherapy. The stratification of such cases by the pre-IFRT CA-125 level and platinum sensitivity status could help to determine the potential benefits of IFRT.
Although more than half of all patients experienced controlled local disease with a median in-field control duration of 18.4 months, approximately 50% of cases in the current study experienced in-field local failure. Due to the lack of an appropriate radiation dose recommendation, we initially started an IFRT for a few patients with a relatively low but safe radiation dose (EQD2 45 Gy). This high local failure rate can be explained by the inclusion of patients in this early era. After early experiences, we elevated the radiation dose more than 50 Gy, and even adopted SBRT. We also observed comparable local control with this dose regimen; this remains below the dose that Brown et al. [
4] recommended, as shown in a previous prospective phase II trial [
6]. Further studies are required to identify the optimal dose for better local control.
In addition, we observed no correlation between in-field local control and the tumor location or size and found no difference in local control with respect to nodal vs. extranodal involvement (2-year IFCR, 41.0% vs. 45.9%, p=0.896). By contrast, Fujiwara et al. [
13] reported better response rates for nodal vs. extranodal sites, and Lee et al. [
19] demonstrated an association of smaller tumors with better in-field local control in another series. In our study, we instead observed a strong correlation between a higher prescribed EQD2 and in-field local control. A retrospective analysis by Choi et al. [
16] also discovered that RT dose more than 50 Gy was correlated with better local control. Furthermore, Yahara et al. [
15] also found achievement of better local control after a median dose of 60 Gy without severe toxicity. Currently available techniques, such as intensity-modulated and stereotactic body RT, enable the administration of highly ablative radiation doses. In this context, Lazzari et al. [
10] reported the efficacy and the safety of stereotactic body RT for oligometastatic ovarian cancer. They reported excellent 2-year local control of 68% with 60% of complete response in 82 patients (156 lesions) with a median dose of 24 Gy in 3 fractions. However, the fact that most failures (90.0%) were outside of radiation field with inferior 3-year PFS (8%) should be interpreted with caution. The high frequency of out-field recurrences might be associated with uncontrolled microscopic disease mainly due to platinum resistance. Firstly, we could infer that it is very important to select the patient who could have clinical benefit from RT. In other words, platinum-sensitive tumors are more appropriate candidates for IFRT than platinum-resistant tumors to avoid short-term early out-field disease progression. Secondly, the new surrogate endpoint such as progression free survival after next line of treatment (PFS2) [
20] might be introduced for identifying the potential role of IFRT in this recurrent setting.
Our study had several limitations of note. First, this was a retrospective analysis, and the results should be reviewed cautiously. Second, this was a single-center study and is therefore subject to bias associated with such studies. Despite these limitations, however, our work provides another analysis of a large pool of retrospective data regarding the effects of IFRT for recurrent EOC.
We demonstrated that IFRT can yield excellent treatment outcomes in patients with recurrent ovarian cancer, irrespective of the administration of cytotoxic chemotherapy. Additionally, patients with a normal CA-125 level and/or platinum-sensitive tumors may be good candidates for IFRT with an adequate radiation dose, irrespective of the peritoneal carcinomatosis status, number of sites, and GTV. Further studies are warranted to elucidate the role of IFRT in the course of treatment for recurrent ovarian cancer.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download