Introduction
Pseudomonas aeruginosa is classified as an opportunistic human pathogen. It is commonly associated with cystic fibrosis, chronic obstructive pulmonary diseases, immunocompromised and AIDS patients. In addition, it causes urinary tract infection, burn and wound infections, blood (bacteremia) and CSF (meningitis) infections and nosocomial infection.1
Infections with P. aeruginosa are difficult to treat not only because it is often associated with multidrug-resistant infectionsbut also it is ableto form biofilm. Due to the extensive use of antibiotics as well as the nature of its cell wall, P. aeruginosa is highly resistant to many antibiotics. Currently, it exhibited resistance to several types of antibiotics. Among 7,452 P. aeruginosa isolates tested in USA, 1,151 (15.4%) and 698 (9.4%) isolates were Multidrug resistance (MDR) and extensive drug resistance (XDR), respectively. Also, high rates of cross-resistance with ceftazidime, meropenem, and piperacillin-tazobactam were reported.2 On the other hand, biofilm producing bacteria such as P. aeruginosa are more resistant to antibiotics. The MIC and MBC of the antibiotics against biofilm is 10 - 1000 times higher than planktonic bacteria.3
P. aeruginosa potential pathogenicity is related to their ability to produce several virulence factors. These virulence factors are mainly regulated by intracellular signaling molecules, such as acyl homoserine lactones (AHLs), called quorum-sensing (QS) system. P. aeruginosa possesses three main QS system namely las, rhl and pqs systems.4 The las system controls the expression of virulence factors such as LasB elastase, LasA protease, exotoxin A and biofilm formation whereas rhl system controls the expression of virulence factors such as LasB elastase, pyocyanin, rhamnolipids and hydrogen cyanide. The third QS system, pqs controls the expression of virulence factors such as biofilm formation and bacterial motility.5
Biofilms are structured communities of attached microbes enclosed in an extracellular polymeric substance (EPS) that is composed of polysaccharides, proteins, DNAs and lipids.6 The formation of biofilm comprises several stages including adhesion stage, colonization stage and maturation stage.7 Once the environmental become favorable, microbes move across the surfaces by means of flagella, adhere to the surface, aggregates and microcolonies are formed. Then, production of quorum sensing signals mediates the maturation of the biofilm.
Biofilm supports the microbes with several vital protective mechanisms. Biofilm producing bacteria such as P. aeruginosa are protected from clearance by the immune system. In addition, it plays a significant role in the pathogenicity of acute and chronic infections.8 The ability of bacteria to form biofilm induces a loss of bacterial susceptibility to antibiotics.9 As a result, higher concentration of antibiotics and/or combinations must be used.10
Generally, drugs that inhibit QS system inhibit biofilm formation and the production of several virulence factors.11 Parallel to the problem of antibiotic resistant in planktonic cells and in biofilm producing bacteria, most of the currently used QS inhibitors are highly toxic,12 and should be avoided. Therefore, finding of new nontoxic agents that could inhibit QS system and biofilm formation has stimulated researchers to do so. This was made through using essential oils (EOs) as easy means for getting lower toxicity and higher bacteriostatic and bactericidal activities.13 Therefore, EOs extracted from Thymbra capitata were selected for the present study. T. capitata is herbal woody plant that is native to Mediterranean region, Europe and Turkey. Traditionally, leaves and stems of T. capitata is commonly used to treat cold, influenza and throat infections.14 There are several reports indicative of a variable biological activity of T. capitata EOs such as antimicrobial, antioxidant, anticancer and anti-inflammatory activities.1516 In particular, T. capitata EOs have been reported with antimicrobial activity against Listeria monocytogenes,17 Enterococcus faecalis,18 Bacillus cereus, Micrococcus flavus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Salmonella enterica Typhimurium,1920 Gardnerella vaginalis,21 Candida spp.,22 Aspergillus spp. and dermatophytes.23
EOs and their components have been reported to impair biofilm formation and to interfere with the QS system.2425 Two previous reports showed that T. capitata EOs possess antibiofilm activity. Palmeira-de-Oliveira et al.,22 reported that T. capitata EOs possess promised inhibitory effect against Candida sp biofilm. Machado et al.,21 found that 0.64 µL/mL of T. capitata EOs exhibited strong antibiofilm activity against Gardnerella vaginalis comparing to carvacrol which showed low antibiofilm activity at the same concentration. To our best knowledge, this is the first report about the antibiofilm and anti-QS activity of T. capitata EOs against P. aeruginosa. The aim of this study was to evaluate the antibiofilm and anti-QS activity of T. capitata EOs against P. aeruginosa. The inhibitory effect of the oil upon targeting variable biofilm formation phases was evaluated against two P. aeruginosa strains; the clinical isolate of beta lactamase P. aeruginosa (BL) strain and the reference strain P. aeruginosa 10145.
Experimental
Plant materials
Thymbra capitata leaves were collected from Aye region, Al-Karak, Jordan in April 2017. The plant was identified according to flora Palaestina, part 3 by Dr. Feryal Al-Khresat (Department of Biology, Mu'tah University, Al-Karak, Jordan). A voucher specimen was deposited in the department of medical laboratory sciences, faculty of science, Mu'tah University, Al-Karak, Jordan. The fresh leaves were air dried at room temperature in the shade, grinded into fine powder, and stored as aliquots at 4℃.
Essential oils extraction
The EOs were extracted by water distillation using simple Clevenger apparatus for 4 h. The EOs were separated from the aqueous phase using diethyl etherand dried over anhydrous sodium sulfate (Na2SO4). The extracted oil was measured as mL and stored as aliquots at 4℃ prior to analysis and use.
Gas chromatography-Mass spectrometry (GC-MS) analysis
The chemical composition of the EOs was determined using GC-MS (ChrompackCP-3800 GC-MS-MS-200 equipped with split-splitless injector). DB-5 GC column (5% diphenyl 95% dimethyl polysiloxane), (30 m × 0.25mm ID, 0.25 µm film thickness) was used to separate the oil components according to the protocol previously described.26 A hydrocarbon mixture of n-alkanes (C8-C20) was analyzed separately by GC-MS using the same column (DB-5) and under the same chromatographic conditions. The compounds were identified by comparison of their retention time to n-alkaline retention times and their similarities to mass spectra database (NIST library) and published reports.
Antibacterial activity
Bacterial strains
The clinical isolate of P. aeruginosa (beta lactamase- BL) was cultured from urine sample obtained from Al Bashir Hospital (Amman-Jordan). This isolate was characterized by BIOMÉRIEUX VITEK® 2 SYSTEM. It was imipenem susceptible (IPM-S 34) but Cefotaxime, ceftazidime, gentamicin, tobramycin, ciprofloxacin, norfloxacin resistant. Also, the reference strain P. aeruginosa 10145 provided by Department of Biology, Mu'tah University, Al-Karak, Jordan, was used.
Disc diffusion method
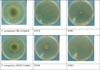
The antibacterial activity using disc diffusion method against P. aeruginosa (BL) and P. aeruginosa 10145 was performed as previously described27 with some modification. Briefly, 1.5 × 108 CFU/mL bacterial suspension was prepared using 0.5 McFarland's standard and 100 µL of this suspension was spread using sterile swab over the surface of Mueller-Hinton agar plates. Then, blank disc impregnated with 5 µL essential oil or Ciprofloxacin (5 µg) placed onto the inoculated plates. After 24 h incubation at 37℃, the zone of inhibition was measured as millimeter diameter. All experiment was performed in triplicate.
Minimum inhibitory concentration (MIC)
The MIC was measured using microdilution method as previously described27 with some modification. Three-fold dilution was prepared using 96 well plat from a stock solution of 10% EOs in DMSO to get 3.33, 1.11, 0.37, 0.123, 0.041, 0.014, 0.0046, 0.0015%. Then, 1.5 × 108 CFU/mL bacterial suspension was prepared using 0.5 McFarland's standard and 10 µL of this suspension was inoculated into each well. The same test was carried out with DMSO as a control. Each experiment was performed in triplicate. The lowest concentration of essential oil needed to inhibit the visible growth of the tested microbes after 24 h was considered as the MIC values.
Biofilm inhibition assay
The anti-biofilm activity of T. capitata EOs was evaluated using crystal violet assay.28 Similar concentrations of MIC wereprepared using 96 well plate. Then 10 µL of bacterial suspension containing 1.5 × 108 CFU/mL (0.5 McFarland's standard) was inoculated into each well. The same test was carried out with DMSO as a control. Each test was performed in triplicate. After 24 h incubation at 37℃, the growth medium was discarded, and the plates were washed and stained with 200 µL of 0.4% crystal violet. After 20 min, the stain was removed, and the excess stain was rinsed off with tap water before adding 200 µL of 95% (v/v) ethanol to solubilize the crystal violet. Then 150 µL from each well were transferred to new 96 well plat for spectrophotometric measurement (OD590 nm) in an ELISA reader.
Anti-quorum sensing activity
Swarming motility assay
Swarming motility assay was performed as previously described.29 Swarm agar plates containing glucose (1%), peptone (0.6%), yeast extract (0.2%) and agar (0.5%), in the absence (control) or presence of 0.041, 0.014, 0.0046% T. capitata EOs were prepared. Overnight culture of P. aeruginosawere gently inoculated at the center of the agar plate and they were incubated at 37℃ in an upright position for 24 h.
Aggregation ability
Aggregation assay was performed according to Shanks et al.,30 Bacterial culture without (control) or with 0.041, 0.014 and 0.0046% T. capitata EOs was incubated at 37℃ for 24 h and the absorbance at 600 nm was measured (OD prevortex). Then, the culture tubes were vortexed for 1 min and the absorbance at 600 nm was measured (OD postvortex). The percent of aggregation was calculated using the following formula: ((OD postvortex − OD prevortex) / OD postvortex)*100
Hydrophobicity
The surface hydrophobicity assay was used to evaluatedthe adhesion of bacteria to hydrocarbon according to Rosenberg et al.31 Briefly, bacteria culture was grown in absence (control) and presence of 0.041, 0.014 and 0.0046% T. capitata EOs. After 2 h at 37℃, the absorbance of the culture was measured at 600 nm and was designed Ai. Then 1.5 ml of the bacterial suspension was mixed with 1.5 ml of butanol and shaken for 2 min. After 15 min, the absorbance of the suspension was measured at 600 nm and was designed Af. The hydrocarbon separation ration (FPc) was used to evaluate the adhesion of the bacteria to the hydrocarbon according to the following formula FPC (%) = 100 * (Ai − Af) / Ai
Pyocyanin inhibition assay
The production of pyocyanin pigment was performed as previously described28 with some modification. Briefly, 7.5 mL cell free supernatant of the test bacteria grown in absence or presence of 0.041, 0.014 and 0.0046% T. capitata EOs was mixed with 4.5 mL chloroform. Then, 3 mL of the organic green-blue layer was collected and mixed with 1.5 mL of 0.2N HClto give pink solution.The pyocyanin acid layer was separated and quantified by measuring the absorbance using spectrophotometer at 520 nm.
Rhamnolipid assay
The amount of rhamnolipids secreted into medium was evaluated using orcinol method according to Luo et al.32 In brief, 3 mL of diethyl ether was mixed with 1ml of cell free supernatant of the test bacteria grown in absence or presence of 0.041, 0.014 and 0.0046% T. capitata EOs. The mixture was vortexed and centrifuged at 10,000 rpm. Then the diethyl ether fraction was separated and evaporated. The dried extract was dissolved in 200 µL H2O and mixed with 900 µL of 0.18% orcinol (w/v) in 53% (v/v) H2SO4. Using water bath, samples were boiled and allowed to cool in dark for 30 min. Then, the absorbance was measured at 421 nm and the percentage of rhamnolipids production inhibition was calculated.
LasA staphylolytic assay
LasA protease activity was performed according to Andrejko el.33 Briefly, overnight culture of S. aureus (50 mL) was boiled for 10 min. The supernatant was removed after centrifugation at 10,000 for 10 min and the resulted pellet was resuspended in 10 mmol Na2PO4. The prepared culture of P. aeruginosa (BL) and P. aeruginosa 10145 in absence or presence of 0.041, 0.014 and 0.0046% T. capitata EOs were centrifuged and 100 µL from the supernatant was mixed with 900 µL of S. aureus solution and the absorbance was measured using spectrophotometer at OD600 nm after 0, 30, 60 and 90 min. Each test was performed in triplicate.
Result and Discussion
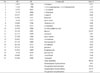
The EOs from the aerial part of T. capitata has been analyzed using GC/MS. As shown in Table 1, 21 compounds were identified representing 90.16% of the total oil. Oxygenated monoterpenes (59.16%) was characterized as a major class of compounds followed by oxygenated sesquiterpenes (15.47%), sesquiterpene hydrocarbons (14.75%) and monoterpenes hydrocarbons (3.01%). The results also show that thymol (23.25%) is the most dominant compound followed by δ-cadinene (8.62%), 1,8-cineole (6.71%), α-terpineol (6.26%) and veridiflorol (5.04%), terpinen-4-ol (4.88%) and cis-chrysanthemic acid (4.88%).
The chemical composition of T. capitata EOs is in agree with previously published studies, which reported oxygenated monoterpens as the major chemical class in this oil. According to “The Essential Oil Database”, the phenolic monoterpens thymol and carvacrol are the major components in T. capitata EOs. Other components such as p-cymene and γ-terpinene could be considered likewise as major components in this species.34 Based on its major components, there are three different T. capitata chemotypes namely; thymol, carvacrol and mixed thymol-carvacrol chemotypes.35 In this study, thymol was the major component; suggesting that this species being fits into the first group. It was reported that T. capitata oil, which was collected from Northern Amman (Amman, Jordan), is oxygenated monoterpens rich oil, mainly thymol (26%) and carvacrol (37%), suggesting that this species is allocated in a mixed thymol-carvacrol type.26 In fact, the composition of the EOs are variable based on several factors such as genetic, environmental condition and harvesting time.3637
The antibacterial activity of EOs of T. capitata was evaluated using disc diffusion method at concentration of 5 µL per disc. As shown in Table 2, the oil exhibited moderate antibacterial activity against P. aeruginosa (BL) and P. aeruginosa 10145 with 10.5 and 12.7mm inhibition zone. The effects of EOs on the inhibition of bacterial growth was determined using a microdilution method at concentrations equal to 3.33, 1.11, 0.37, 0.123, 0.041, 0.014, 0.0046, 0.0015%. At the highest concentrations tested (3.33 and 1.11%), the EOs has completely inhibited the visible growth of both strains tested and 1.11% was reported as the MIC (Table 2). At concentrations lower than 0.123%, there were no inhibition effect and the growth of P. aeruginosa (BL) and P. aeruginosa 10145 were similar to the control group. Further, sub-MIC concentrations (0.041, 0.014 and 0.0046%) were used in the experiments.
The activity of T. capitata EOs against P. aeruginosa strains was in accordance to previous reports. Džamić et al.38 found that the MIC of T. capitata EOs against P. aeruginosa is equal to 1 µg/mL whereas Neves et al.37 found it equal to 7.5 µg/ml. In a study performed by Al Hafi et al.36, the MIC of T. capitata against P. aeruginosa was determined to be 0.4 mg/mL. The bacteriostatic effect at low concentration (1.11%) indicated the potent antibacterial activity of T. capitata EOs.
The antibacterial activity of EOs is due to the presence of phenolic or aromatic oxygenated compounds.39 Therefore, the result of this study suggested that this potent activity is linking to most dominant compound thymol. Several reports showed that thymol and its isomer carvacrol are the most potent antimicrobial components in EOs.4041 Other components including eugenol, p-cymene and gamma terpinene are also active against several bacterial species.36,39 Also, the possible synergistic effect of the oil components could plays a significant role in inhibition of the bacterial growth.39 The mode of action of these components probably through membrane damage. Losing of membrane integrity lead to spill out the cell components and death.42
The inhibitory effect of T. capitata EOs on biofilm formation of the clinically isolated P. aeruginosa (BL) and P. aeruginosa 10145 was evaluated using crystal violet assay. As shown in Fig. 1, T. capitata EOs exhibited remarkable inhibition activity on the biofilm formation in a dose-dependentmanner. When the concentration of the EOs was 0.0046%, the inhibition activities on the biofilm formation of P. aeruginosa (BL) and P. aeruginosa 10145 were 44.8% and 49.8%, respectively. The highest concentration tested (0.041%), completely inhibited the biofilm formation of P. aeruginosa (BL) and P. aeruginosa 10145 by 99.6% and 98.4%, respectively.
It was found that adhesion of planktonic bacteria to surface is the first and most crucial step in biofilm formation. Adhesion behavior has been correlated with the swarming motility, the aggregation ability and hydrophobicity.4344 Alterations in one of these processes could subsequently affect the other. Thus, the inhibitory effect of T. capitata EOs on swarming motility, aggregation ability and hydrophobicity was evaluated.
The swarming motility of the tested strains was evaluated using 0.5% agar (Fig. 2). Comparing with the control group, increasing the concentration of T. capitata EOs from 0.014 to 0.041% resulted in remarkable inhibition of the swarming motility of P. aeruginosa (BL) and P. aeruginosa 10145.
Swarming motility plays a significant role in biofilm formation. It allowed bacteria to aggregate, colonize, and then facilitate the initiation of adhesion. Also, it has a role in protecting bacteria from harmful substances such as antibiotics and immune system molecules.45 In the present study, EOs of T. capitata effectively reduced the swarming motility and the aggregation ability, thus inhibiting the formation of biofilms in the clinically isolated strain and the reference strain. Our results are similar to that of Lee et al.46 who reported significant inhibitory effect on swarming motility of Escherichia coli when treated separately with 0.01% of oregano oil, thyme oil, carvacrol or thymol.
As shown in Fig. 3, the percentage of aggregation was remarkably reduced when P. aeruginosa (BL) and P. aeruginosa 10145 treated with different concentrations of T. capitata EOs. The percentage of aggregation was EOs concentration dependent. For example, at 0.0%, the aggregation percentage of P. aeruginosa (BL) and P. aeruginosa 10145 was 46.4 and 41.1%, respectively, while at the highest concentration tested (0.041%) the aggregation percentage was 27.3% and 26.6%.
The Effect of different concentrations of T. capitata EOs on the hydrophobicity of P. aeruginosa (BL) and P. aeruginosa 10145 using hydrocarbon separation ratio (FPc) is shown in Fig. 4. The result shows that T. capitata EOs can reduce the hydrophobicity of P. aeruginosa (BL) and P. aeruginosa 10145 in dose dependent manner. When the concentration of EOs changed from 0 to 0.041%, the FPc of P. aeruginosa (BL) and P. aeruginosa 10145 dropped from 44.6 and 62.6% to 27.2 and 45.2%, respectively.
In this study, the hydrophobic ability test for P. aeruginosa was made to evaluate the reducing of adhesion ability by T. capitata EOs. The results showed that T. capitata EOs decreased the hydrophobicity of the tested bacteria. The reduction of the hydrophobicity led to minimizing the bacterial adhesion and in turn inhibiting additional biofilm formation. Koraichi Saad et al.47 reported significant decrease in adhesion ability of P. aeruginosa (up to 91%) in the presence of carvacrol or thymol at low MIC of 0.00125%.
Three secreted virulence factors that regulated by QS system have been evaluated in this study including pyocyanin, rhamnolipids and LasA protease. The reduction in the production of these factors indicates the ability of anti-QS by T. capitata EOs.
As shown in Fig. 5, the production of pyocyanin decreased with increasing the T. capitata EOs concentrations. Significant reduction in the absorption of pyocyanin produced by P. aeruginosa (BL) and P. aeruginosa 10145 at 520 nm from 0.57 and 0.71 (control groups) to 0.189 and 0.256 (0.041%), respectively.
Pyocyanin is a particularly effective virulence factor produced by P. aeruginosa because of its broad spectrum against variable cellular components and metabolisms including electron transport chain, ions transport and cell growth.48 It is a zwitter-ion that can pass through the biological membrane easily. Therefore, it serves as a mobile electron carrier that might affects many membranes functions such as ions homeostasis (Ca), ATP production and subsequently inhibit many Ca- and energy-dependent cellular metabolisms.49 In addition, pyocyanin has been reported to inhibit lymphocyte proliferation, epidermal cell growth and prostacyclin release.50 Accordingly, it serves to facilitate the pathogenicity and maximize the harmful effect to host cells. The EOs of T. capitata remarkably reduced the production of pyocyanin in P. aeruginosa. Tapia-Rodriguez et al.42 reported a significant decrease in the production of P. aeruginosa pyocyanin to 60% when treated with 3.9 mmol/L of carvacrol.
The effect of T. capitata EOs on rhamnolipid production in P. aeruginosa (BL) and P. aeruginosa 10145 was evaluated using orcinol method. The decrease in the production of rhamnolipid with increasing the concentrations of T. capitata EOs was observed (Fig. 6). At low concentration tested (0.0046%), the percentage of rhamnolipid production inhibition in P. aeruginosa (BL) and P. aeruginosa 10145 was 23.5 and 18.3%, respectively, whereas at the highest concentration tested (0.041%) the percentage was 75.9% and 77.8%.
Rhamnolipids play a significant role in biofilm formation in P. aeruginosa. The production of rhamnolipid behaves as a wetting agent that facilitates swarming motility. Also, rhamnolipids is effective signals for the biofilm maturation through maintaining the macrocolonies shape and facilitating the opening of channels in a mature biofilm.51
LasA and LasB proteases serve as invasion factors in P. aeruginosa. Their role include disruption of tight junctions of epithelial cells and subsequent alterations to cell polarity.52 In addition, they can decrease toxins level and thus reduce inhibition of invasion. In this study, T. capitata EOs caused rapid reduction in staphylolytic protease activity of the P. aeruginosa (BL) and P. aeruginosa 10145. As shown in Fig. 7, dose dependent reduction in protease activity was reported for both strains tested. Moreover, rapid reduction in protease activity was reported during the first 60 min and the highest reduction was observed for P. aeruginosa (BL) at 0.37%. In another study, a significant reduction in LasA protease activity by 63.1% in Pseudomonas aeruginosa treated with Murrayakoenigii EOs at a concentration of 0.3% was recently reported.53
Studies have showed that EOs and their major components exhibited anti QS and antibiofilm activities. The EOs of tea tree (Melaleuca alternifolia), rosemary (Rosmarinus officinalis),54 Piper bredemeyeri, Piper bogotense, Piper brachypodom55 and Lippia alba56 have been proved to inhibit QS activity on Chromobacteriumviolaceum. Many of EOs containing thymol or carvacrol showed anti QS activities, in which these activities is compatible with the current study. Significant anti QS activity was reported for the oregano EOs at concentrations as low as 0.0156, 0.0312, 0.0625, and 0.125 mg/mL against C. violaceum.57 A recent study showed that the inhibition of biofilm formation by carvacrol rich Thymus daenensis EOs was more potent than thymol rich Saturejahortensis EOs at sub-MIC (0.0312, 0.0156, 0.0078 and 0.0039 µl.mL-1).58 Furthermore, carvacrol, the major component of oregano EOs, was reported with potent anti-QS activity at less than 0.05 mM.59 In another study conducted by Woertman,60 carvacrol inhibited QS activity at concentrations equal to 0.2 and 0.4 mM. Myszka et al.61 found that EOs of Thymus vulgare and its major components carvacrol and thymol at sub MIC (20, 2.0 and 4.0 µL per mL, respectively) significantly inhibited QS activity and bacterial motility, suppressed flagella gene expression and inhibited the biofilm formation of P. fluorescens.
In conclusion, T. capitata EOs has remarkably inhibited the biofilm formation as well as the QS virulence factors in beta lactamase producing P. aeruginosa. Considering their traditional values, broad spectrum antibacterial activity and their multiple mode of action, EOs of T. capitata may be a promising agent for curing P. aeruginosa infections. Further studies are required toisolate the components of T. capitata EOs and to evaluate the synergism and antagonism between these components.




 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download