Abstract
Although the functions of a standardized extract of Gingko biloba leaves (EGb 761®) has been reported with regard to neurobiological properties, no attention has been paid to the impact of EGb 761® on the neuronal regulation of energy homeostasis. To evaluate the hypothesis that EGb 761® affect the secretion of peptide tyrosine tyrosine (PYY) and the activation of free fatty acid receptor 4 (FFA4), which are involved in the neuronal circuitries that control energy homeostasis by inducing the transfer of information about the influx of energy to the brain, we examined whether EGb 761® can stimulate PYY secretion in the enteroendocrine NCI-H716 cells and if EGb 761® can activate FFA4 in FFA4-expressing cells. In NCI-H716 cells, EGb 761® stimulated PYY secretion and the EGb 761®-induced PYY secretion was involved in the increase in intracellular Ca2+ concentration and the activation of FFA4. Furthermore, in FFA4-expressing cells, EGb 761® activated FFA4. These results suggest that EGb 761® may affect the control of energy homeostasis via the regulation of PYY secretion and FFA4 activation.
Recently, considerable progress has been made in understanding how nutrient-induced peripheral signals are integrated in the neuronal circuitries that control energy homeostasis. The circuitries require the transfer of accurate information regarding the influx of energy to the brain. This is accomplished by nutrient sensor mechanisms distributed along the gastrointestinal (GI) tract. Throughout the GI tract, specialized endothelial cells called enteroendocrine cells (EECs) sense the presence of luminal nutrients and consequently release hormones or neuropeptides which convey this information to the brain either directly through the circulatory system and by affecting sensory neurons that project to the brain.1
Peptide tyrosine tyrosine (PYY), one of GI hormones and neuropeptides, acts through the circulation and acts on neuropeptide Y (NPY) receptors within the arcuate nucleus of the hypothalamus (ARC) to decrease expression of agouti-related peptide (AgRP). PYY inhibits upper GI motility, mucosal fluid and electrolyte secretion as well as colonic transit, which together promotes satiety, increases satiation, suppresses appetite, prevents obesity and regulates energy homeostasis.2 PYY is secreted from the EECs in response to fatty acids, glucose and amino acids. Of these, long-chain fatty acids (LCFAs) derived from dietary lipids are the most potent stimulants of PYY release.3
Luminal LCFAs are sensed by free fatty acid receptor (FFA) 4, previously known as G protein-coupled receptor (GPR) 120, located on the EECs in the proximal and distal intestine.4 FFA4 has been reported to participate in the peripheral control of energy homeostasis.5678 FFA4 has been shown to be co-expressed in neuropeptide Y-expressing neurons in the arcuate nucleus,910 and thus, the activation of FFA4 in the hypothalamus could directrly control physiological processes associated with energy homeostasis, including gastrointestinal peptide hormone secretion, islet function, food preference, appetite control, adipogenic differentiation, insulin sensitization and anti-obesity effects. FFA4 has been reported to control energy homeostasis peripherally and via direct central actions.9 FFA4 mediates PYY secretion from the EECs.1112 Naturally occurring dietary LCFAs, including α-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, have been identified as FFA4 agonists.13
Non-nutrients and natural products can target the secretion of PYY and the activation of FFA4. There have been a few reports showing that natural products induce PYY secretion in vitro and in vivo. (−)-Epigallocatechin-3-gallate, from green tea, has been shown to induce PYY secretion in Caco-2 cells.14 Methyl syringate, an ingredient in Kalopanax pictus, has been shown to suppress food intake and delay gastric emptying via elevated plasma PYY levels in mice.15 Dietary administration of the freeze-dried leaves from nasturtium (Tropaeolum majus L.) plants containing glucotropaeolin increased the serum level of PYY in male subjects.16 The consumption of polyphenol-rich beverages made from turmeric (Curcuma longa) before intake of a carbohydrate challenge has been reported to increase serum levels of PYY in healthy subjects.17
There are only a few reports of natural FFA4 agonists. Grifolic acid and grifolic acid methyl ether, isolated from Albatrellus dispansus, acted as FFA4 agonists in a cell line stably expressing human FFA4.18
A standardized extract of Ginkgo biloba L. leaves (EGb 761®) is among the most widely used natural product in the world. Scientifically, the beneficial effects of EGb 761® have been studied in the context of treating neural disorders, such as cognitive deficits, Alzheimer's disease, memory loss, geriatric complaints, such as vertigo, and psychiatric impairments, like schizophrenia.192021 Based on the aforementioned literature which supports the neurobiological effects of EGb 761®, we hypothesized that EGb 761® might influence PYY secretion and FFA4 activation, which induce the transfer of information about the influx of energy to the brain. To evaluate this hypothesis, we investigated: (1) whether EGb 761® stimulates PYY secretion and intracellular Ca2+ mobilization in NCI-H716 cells, if NCI-H716 cells express FFA4, and if the transfection with FFA4-specific small interference RNA affects PYY secretion and intracellular Ca2+ changes induced by EGb 761® in NCI-H716 cells, and (2) if EGb 761® activates FFA4 in HEK 293 cells stably transfected with human FFA4.
EGb 761® which are approved by the Korea Food and Drug Administration was kindly provided by YuYu Pharma Inc. (Seoul, Korea). The EGb 761® provided (lot No. 29007583) contained 25.5% Ginkgo flavone glycosides and 9.2% terpene lactones according to the test report analysed by YuYu Pharma Inc. RPMI1640 medium, Dulbecco's modified Eagle medium (DMEM), high-glucose DMEM, fetal bovine serum (FBS), penicillin, streptomycin, L-glutamine and trypsin-ethylenediaminete traacetic acid (EDTA) were purchased from Gibco Co. (Grand Island, NY, USA). Matrigel was from Becton-Dickinson Co. (Bedford, MA, USA). A commercially available FFA4 agonist [FFA4 agonist III; 3-(4-((4-fluoro-4′-methyl-(1,1′-biphenyl)-2-yl)methoxy)-phenyl) propanoic acid] was from Calbiochem (Darmstadt, Germany). PYY active ELISA kits were from Millipore (Billerica, MA, USA). FLIPR Calcium 5 Assay Kit was from Molecular Devices (Sunnyvale, CA, USA). EGb 761® and water-insoluble chemicals were first dissolved in dimethyl sulfoxdie (DMSO) and diluted to a final concentration of 0.1% (v/v) DMSO in cells.
Human NCI-H716 cells were obtained from American Type Culture Collection (ATCC No. CCL-251; Manassas, VA, USA), and cultured according to the method described previously.2223 Briefly, the cells were grown in RPMI 1640 supplemented with 10% (v/v) FBS, 2 mM L-glutamine, 100 IU/mL penicillin and 100 mg/mL streptomycin at 37 ℃ in 5% CO2.
Human Free Fatty Acid Receptor 4 cells (FFA4 cells), which are human embryonic kidney (HEK) 293 cells stably transfected with human FFA4 and Gα16, were purchased from Charles River Laboratories Inc. (San Diego, CA, USA). The cells were cultured in DMEM supplemented with 10% (v/v) FBS, 100 IU/mL penicillin and 100 mg/mL streptomycin at 37 ℃ in 5% CO2.
HEK 293 cells were obtained from ATCC (No. CRL-1573), and cultured as described in the culture of FFA4 cells.
The cytotoxic effects of test agents on NCI-H716, FFA4 and HEK 293 cells were examined by measuring the release of lactate dehydrogenase (LDH; a stable cytosolic enzyme) into the supernatant of the cells exposed to test agents, respectively. The cell densities plated in microplates were same as in intracellular Ca2+ measurements in the cells. The differentiation time before the addition of test agents was same as in the PYY secretion assay in NCI-H716 cells. The incubation time with test agents was 1 h in the cells. The cell-free culture supernatant was collected and incubated with the reaction mixture from LDH cytotoxicity detection kit (Clontech laboratories, California, USA). Cytotoxicity was calculated as the relative release (%) of LDH after exposure to test agents compared the total LDH (100%) released upon treatment with a lysis reagent.24
Endocrine differentiation was induced by seeding the cells at a density of 5.0 × 104 cells/well in 96-well microplates coated with matrigel in high-glucose DMEM, 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin and 100 mg/mL streptomycin for 48 h. Then, medium was replaced by Krebs-Ringer bicarbonate buffer (KRB, 128.8 mmol/L NaCl, 4.8 mmol/L KCl, 1.2mmol/L KH2PO4, 1.2 mmol/L MgSO4, 2.5 mmol/L CaCl2, 5 mmol/L NaHCO3, 10 mmol/L HEPES, and 0.2% BSA, pH 7.4) containing test agents. Following incubating at 37 ℃ for 1 h, the supernatants were collected for PYY measurement using ELISA kits according to the manufacturer's instructions. The values of PYY were expressed as fold of the untreated control.
The cells were seeded at 5.0 × 104 cells/well in 96-well clear-bottomed black plates (Corning Inc., Bedford, MA, USA) precoated with matrigel for 48 h at 37 ℃. Then the cells were loaded with the calcium-sensitive dye from the Calcium 5 Assay kit for 1 h at 37 ℃. Then, the cells were stimulated with test reagents in Hank's balanced salt solution (HBSS). Upon stimulation, intracellular Ca2+ released can bind to the dye and alter its fluorescence intensity. This change in fluorescence signal, and thus the flux in the concentration of intracellular Ca2+ ([Ca2+]i), is detected and quantitated by fluorescence imaging using a FLIPR reader. Fluorescence values of excitation at 485 nm and emission at 525 nm were monitored at 2-sec intervals for 120 sec using a FLIPR reader FlexStation 3 (Molecular Devices) at 25 ℃. The values of each well were detected and results were calculated as ΔRFU (delta relative fluorescent units), i.e., (maximum fluorescent value) − (minimum fluorescent value). The data are reported as the mean ± SEM of the ΔRFU.22
This in vitro assay serves to assess agonistic activity of test agents against the human FFA4. The assay utilized HEK 293 cells stably transfected to express the human FFA4 (FFA4 cells; Charles River Laboratories Inc.). FFA4 and HEK 293 cells were seeded at 2.0 × 104 cells/well in 96-well clear-bottomed black plates (Corning Inc.) for 48 h at 37 ℃, respectively. Then the cells were loaded with the calcium-sensitive dye from the Calcium 5 Assay kit for 1 h at 37 ℃, respectively. Then, the following procedures were as same as described in Calcium flux assay in NCI-H716 cells.
The extraction of total RNA, cDNA synthesis, and PCR reactions were performed as described previously.22 The primer sequences used here were as follows: FFA4 forward 5′-gtcattgtgatcagttactccaaaa-3′, reverse: 5′-act gtgagtcacgacagctctt-3′; GAPDH forward 5′-agccacatcgctc agacac-3′, reverse: 5′-gcccaatacgac caaatcc-3′. GAPDH mRNA was used as an endogenous control to normalize expression level.
The validated small interference RNA (siRNA) duplexes against FFA4 (Cat. No. 1064123) and a scrambled negative control siRNA were obtained from Bioneer (Daejeon, Korea). Transfection with siRNA duplexes were performed with lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Gene knockdown was verified by real time PCR 48 h after the transfection. After the cells were transfected with siRNA, the cells were differentiated for 48 h before the functional assays of PYY secretion and calcium mobilization were performed.
Experiments were performed at least three separate experiments. All values are expressed as mean and standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey's test and Student's t-test was used for statistical analysis (IBM SPSS statistics 20 software, IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
To examine whether test agents induced cell lysis, LDH release was measured in NCI-H716, FFA4, and HEK 293 cells. EGb 761® at concentrations up to 500 µg/mL, or FFA4 agonist III up to 200 µM were applied to NCI-H716, FFA4, and HEK 293 cells for 1 h. None of the tested agents induced LDH release (data not shown). Therefore, we added EGb 761® at a concentration of 500 µg/ml, FFA4 agonist III at 200 µM for subsequent experiments using NCI-H716, FFA4, and HEK 293 cells.
NCI-H716 cells, a human EEC cell line, have been reported to secret PYY in response to an agonist of the bitter taste receptors.22 Meanwhile, the activation of FFA4 mediates PYY secretion from EECs in the proximal and distal intestine of humans and animals.12 We therefore explored PYY release in response to an FFA4 agonist. As expected, the application of 100 µM FFA4 agonist III, a synthetic agonist and a positive control, to NCI-H716 cells increased the secretion of PYY 2.4-fold when compared with the untreated control (p < 0.05; Fig. 1A). This result indicates that the NCI-H716 cell line is a feasible model for studying PYY secretion.
The ability of EGb 761® to induce PYY release from NCI-H716 cells was examined. Treatment with EGb 761® for 1 h resulted in a significant (P < 0.05), and dosedependent increase in the secretion of PYY (1.4-fold at 100 µg/mL, 2.4-fold at 250 µg/mL, and 3.6-fold at 500 µg/mL) compared to the untreated control (Fig. 1A). The half maximal effective concentration (EC50) of EGb 761® for PYY release was 56.2 µg/mL, when the response to 100 µM FFA4 agonist III was regarded to be the maximum response.
The increases in [Ca2+]i might be responsible for the PYY secretion induced by EGb 761® in NCI-H716 cells. Application of 100, 250, and 500 µg/mL of EGb 761® and FFA4 agonist III significantly induced an increase in [Ca2+]i compared to the untreated control in the cells (Fig. 1B). The EC50 of EGb 761® for an increase in [Ca2+]i was 152.6 µg/mL, when the response to 100 µM of FFA4 agonist III was regarded to be the maximum response.
The expression of genes encoding FFA4 has been reported in human and rodent EECs, and in a murine cell line, STC-1 cells.25 However, the expression of FFA4 in NCI-H716 cells has not been confirmed previously. We analyzed the expression level of FFA4 mRNA normalized to the level of GAPDH mRNA using quantitative RT-PCR analysis in NCI-H716 cells. In the study we detected the expression of FFA4 mRNA (Fig. 2A).
To determine the involvement of FFA4 on EGb 761®-induced PYY secretion and [Ca2+]i increase in NCI-H716 cells, we transfected these cells with FFA4-targeted siRNA. We observed that the cells transfected with 1, 10 and 100 nM of FFA4-targeted siRNA reducted FFA4 mRNA by 23.4, 48.8 and 74.3%, compared with cells transfected with non-targeted siRNA (Fig. 2B). Based on these results, 100 nM siRNA was used in all further experiments performed to determine the involvement of FFA4 in EGb 761®-induced PYY release. When 100 nM of FFA4 siRNA was applied, PYY secretion induced by 100 µM of FFA4 agonist III was significantly inhibited by 33.9% (Fig. 2C), and the increase in [Ca2+]i was decreased by 53.2% (Fig. 2D). PYY secretion induced by EGb 761® at 100, 250 and 500 µg/mL significantly decreased by 36.9, 36.5 and 25.0%, respectively, with FFA4 siRNA treatment (Fig. 3C); also, [Ca2+]i increase induced by EGb 761® at 100, 250 and 500 µg/mL was significantly decreased by 110, 67.8 and 40.1%, respectively, in cells transfected with FFA4-targeted siRNA (Fig. 3D). Our results indicated that the EGb 761®-induced PYY secretion and [Ca2+]i increase occurred in an FFA4-dependent manner.
To investigate whether EGb 761® activates FFA4, we tested for its ability to induce elevation of [Ca2+]i in HEK 293 cells stably transfected with human FFA4. Treatment with EGb 761® and FFA4 agonist III in FFA4 cells significantly increased [Ca2+]i compared to the treatment in HEK 293 cells that were not transfected with FFA4 (Fig. 3A and 3B). EGb 761® increased [Ca2+]i in a dosedependent manner at the concentrations from 50 to 500 µg/mL (Fig. 3A). EGb 761® displayed a half maximal effective concentration (EC50) of 151.4 µg/mL when [Ca2+]i increase caused by treatment with 100 µM of FFA4 agonist III was regarded as the maximum response (Fig. 3B).
There have been no reports demonstrating that EGb 761® has effects involved in the control of energy homeostasis. In this study, EGb 761® induced the secretion of PYY, which mediates the transfer of information about the influx of energy to the brain. The result suggests that EGb 761® may affect satiation, food intake and obesity by its influence on the neuronal circuitries of energy homeostasis. Furthermore, EGb 761® activated FFA4, which also is involved in the neuronal circuitries of energy homeostasis. The result suggests that EGb 761® may affect GI hormone secretion, type 2 diabetes mellitus and obesity by its effect on the neuronal circuitries that govern energy homeostasis. Additionally EGb 761® may have undiscovered biological properties associated with PYY secretion and FFA4 activation.
It was shown that EGb 761® induced PYY secretion and [Ca2+]i increase in NCI-H716 cells in the results. The results suggest that the increase in [Ca2+]i might be involved in EGb 761®-induced PYY secretion. The molecular pathways coupling nutrients to PYY secretion depend on the nutrient type but involve the increase in [Ca2+]i and/or the change in the level of intracellular cyclic Adenosine Monophosphate (cAMP). The increase in [Ca2+]i and the change in cAMP level is induced by the activation of G-protein coupled receptors (GPCRs). The GPCRs which are involved in the stimulation of PYY secretion in EECs have been reported to be the sweet taste receptors (T1R1-T1R3), D-amino acid sensors, the fatty acid receptors (FFAs; FFA1, FFA2, FFA3 and FFA4), L-amino acid sensors, the calcium-sensing receptor (CaSR), the oleoylethanolamide (OEA) receptor (GPR119) and the bile acid receptor (TGR5, also known as G protein-coupled bile acid receptor 1, GPBAR1). T1R1-T1R3 and D-amino acid sensors are known to signal through α-gustducin, which is coupled to increases in Ca2+ influx through voltage gated ion channels or mobilization of intracellular Ca2+ stores. FFAs, L-amino acid sensors and CaSR are known to signal through Gq, which is also coupled to increases in Ca2+ influx. Both GPR119 and TGR5 are known to be Gs-coupled receptors, which is coupled to the cytoplasmic elevation of cyclic Adenosine Monophosphate (cAMP) concentration in EECs.12 The stimulation/activation of FFA4 by FFA4 agonists triggers the mobilization of intracellular Ca2+, which then induces the release of PYY from EECs.26 From the results that the transfection with FFA4-targeted siRNA inhibited EGb 761®-induced PYY secretion and [Ca2+]i increase in NCI-H716 cells, it was suggested that FFA4 was involved in EGb 761®-induced PYY secretion and [Ca2+]i increase in the cells. However, the above-mentioned receptors except FFA4 with α-gustducin-, Gq- and Gs-coupled pathways could be responsible for the EGb 761®-induced PYY secretion and [Ca2+]i increase in the cells.
FFA4, which functions as a receptor for LCFAs, has been reported to participate in the regulation of various physiological processes, including gastrointestinal peptide hormone secretion, islet function, food preference, appetite control, anti-inflammatory effects, adipogenic differentiation, insulin sensitization and osteoclastogenesis. FFA4 has also been associated with the regulation of appetite, the release of insulin controlling hormones, insulin sensitization, anti-inflammatory effects, and anti-obesity effects.5678 Together, these data suggest that the beneficial effects of EGb 761® may be due in part to the activation of FFA4.
Further researches remain to be demonstrated which components of EGb 761® are responsible to the EGb 761®-induced PYY secretion and FFA4 activation, and which GPCRs except FFA4 are contributed to the EGb 761®-induced PYY secretion.
Figures and Tables
 | Fig. 1EGb 761®-induced PYY secretion and [Ca2+]i increase in NCI-H716 cells. (A) EGb 761®-induced PYY secretion in NCI-H716 cells. (B) EGb 761®-induced [Ca2+]i increase in NCI-H716 cells. Values are expressed as mean ± SEM from three separate experiments performed in triplicate. Bars not sharing the same letters differ significantly (p < 0.05) by Tukey's test. |
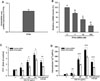 | Fig. 2FFA4-mediated PYY secretion and [Ca2+]i increase induced by EGb 761® in NCI-H716 cells. (A) Real-time RT-PCR analysis of FFA1, FFA2, FFA3, FFA4, and GPR119 mRNA expression in NCI-H716 cells. (B) FFA4 mRNA expression by transfection with 1, 10, and 100 nM FFA4-targeted siRNA in NCI-H716 cells. (C) Effect of transfection with 100 nM FFA4-targeted siRNA on EGb 761®-induced PYY secretion in NCI-H716 cells. (D) Effect of transfection with 100 nM FFA4-targeted siRNA on EGb 761®-induced [Ca2+]i increase in NCI-H716 cells. Values are mean ± SEM from three separate experiments performed in triplicate. Bars not sharing the same letters differ significantly (p < 0.05) by Tukey's test. *p < 0.05; **p < 0.01; ***p < 0.001 vs. groups treated with non-targeted (control) siRNA. |
 | Fig. 3[Ca2+]i response induced by (A) EGb 761® and (B) FFA4 agonist III in FFA4 cells (HEK 293 cells stably expressing FFA4 and G16). Values are expressed as mean ± SEM from three separate experiments performed in triplicate. ***p < 0.001 vs. groups treated with EGb 761® or FFA4 agonist III in HEK 293 cells. Bars not sharing the same letters differ significantly (p < 0.05) by Tukey's test. |
Acknowledgments
This work was supported by a grant from Small Grant for Exploratory Research of Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number 2016R1D1A1A02937328), and a grant form Main Research Program funded by Korea Food Research Institute (grant number E0111203-05).
The authors thank Dr. Hyun-no Cho and Ms. Na-yeon Kim for their assistances in in preparing the manuscript
References
1. Clemmensen C, Müller TD, Woods SC, Berthoud HR, Seeley RJ, Tschöp MH. Cell. 2017; 168:758–774.
2. Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj J, Bell JD, Bell-Anderson K, Mackay CR, Ghatei MA, Bloom SR, Frost G, Bewick GA. Mol Metab. 2016; 6:48–60.
3. Lousie P, Herbert H. Kastin AJ, editor. Handbook of Biologically Active Peptides (Second Edition): PYY. United States of America: Academic press;2013. p. 1307–1313.
4. Depoortere I. Peptides. 2015; 66:9–12.
5. Im DS. Mol Aspects Med. 2018; 64:92–108.
6. Gribble FM, Diakogiannaki E, Reimann F. Handb Exp Pharmacol. 2017; 236:181–203.
7. Hansen SV, Ulven T. Handb Exp Pharmacol. 2017; 236:33–56.
8. Liu HD, Wang WB, Xu ZG, Liu CH, He DF, Du LP, Li MY, Yu X, Sun JP. Eur J Pharmacol. 2015; 763(Pt. B):160–168.
9. Auguste S, Fisette A, Fernandes MF, Hryhorczuk C, Poitout V, Alquier T, Fulton S. Int J Neuropsychopharmacol. 2016; 19:1–10.
10. Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. PLoS One. 2012; 7:e30571.
11. Moodaley R, Smith DM, Tough IR, Schindler M, Cox HM. Br J Pharmacol. 2017; 174:4508–4522.
12. Cox HM. Curr Opin Pharmacol. 2016; 31:50–56.
13. Moniri NH. Biochem Pharmacol. 2016; 110-111:1–15.
14. Song WY, Aihara Y, Hashimoto T, Kanazawa K, Mizuno M. J Clin Biochem Nutr. 2015; 57:164–169.
15. Kim MJ, Son HJ, Song SH, Jung M, Kim Y, Rhyu MR. PLoS One. 2013; 8:e71603.
16. Schiess S, Platz S, Kemper M, Schreiner M, Mewis I, Rohn S, Bumke-Vogt C, Pivovarova O, Pfeiffe AFH. Mol Nutr Food Res. 2017; 61:1600886.
17. Zanzer YC, Plaza M, Dougkas A, Turner C, Björck I, Östman E. J Funct Food. 2017; 35:574–583.
18. Hara T, Hirasawa A, Sun Q, Sadakane K, Itsubo C, Iga T, Adachi T, Koshimizu TA, Hashimoto T, Asakawa Y, Tsujimoto G. Naunyn-Schmiedeberg's Arch Pharmacol. 2009; 380:247–255.
19. Mahadevan S, Park Y. J Food Sci. 2008; 73:R14–R19.
20. Ramassamy C, Longpré F, Christen Y. Curr Alzheimer Res. 2007; 4:253–262.
21. Hsieh SK, Chung TY, Li YC, Lo YH, Lin NH, Kuo PC, Chen WY, Tzen JT. J Ethnopharmacol. 2016; 193:237–247.
22. Kim K, Park M, Lee YM, Rhyu MR, Kim HY. Arch Pharm Res. 2014; 37:1193–1200.
23. Reimer RA, Darimont C, Gremlich S, Nicolas-Méttral V, Rüegg UT, Macé K. J Endocrinology. 2001; 142:4522–4528.
24. Nakajima S, Hira T, Yahagi A, Nishiyama C, Yamashita T, Imagi J, Hara H. Mol Nutr Food Res. 2014; 58:1042–1051.
25. Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Nat Med. 2005; 11:90–94.
26. Stewart JE, Feinle-Bisset C, Keast RS. Prog Lipid Res. 2011; 50:225–233.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download