Abstract
The study was conducted to investigate the acute and sub-acute toxicity effect of Aquilaria malaccensis leaves aqueous extract (AEAM) towards male ICR mice in terms of body weight, relative organ weight, mortality rate and sperm parameters. In acute toxicity study, a single dose at of 2000 mg/kg was performed. In sub-acute toxicity study, the mice were received normal saline (control group), 50, 100, 150, 200,500, or 1000 mg/kg of AEAM orally for 21 days of treatment. In sub-acute toxicity study, the number of abnormal sperm were significantly decreased in AEAM 100, 150, 200, 500, and 1000 when compared to the control group. While, the motility of sperm were found to be significantly increased in AEAM 100, 150, 200, and 1000 as compared to the control group. No mortality was recorded in the control group and treated groups in both toxicity studies except for one mouse from AEAM 1000 group. However, the mild sedative effect in terms of the tendency to sleep was clearly noticeable in both toxicity studies. Results indicated that the AEAM can be one of the useful alternative medicine to enhance fertility rate by increasing healthy sperm production.
Go to : 
REFERENCES
(1). Redzuan Nul Hakim A. R., Muhammad Lokman M. I., Afzan M. Y., Azantee Yazmie A. W., Hussin M., Roszaman R. J.Biotechnol. Strategic Health Res. 2017; 1:17–23.
(2). Parandin R., Yousofvand N., Ghorbani R.Iran J. Reprod. Med. 2012; 10:355–362.
(3). Agnes V. F., Akbarsha M. A.Food Chem. Toxicol. 2003; 41:119–130.
(4). Chauhan N. S., Dixit V. K.Int. J. Appl. Res. Nat. Prod. 2008; 1:26–31.
(5). Ekaluo U. B., Erem F. A., Omeje I. S., Ikpeme E. V., Ibiang Y. B., Ekanem B. E.IOSR J. Environ. Sci. Toxicol. Food Technol. 2013; 3:21–23.
(6). Wahab N. A., Mokhtar N. M., Halim W. N., Das S.Clinics. 2010; 65:93–98.
(7). Mahmoud A. S. F., Noor M. M.AIP Conf. Proc. 2013; 1571:227–233.
(8). Nor-Raidah R., Mahanem M. N.Malays. Appl. Biol. 2015; 44:125–131.
(9). Hakim P., Sani H. A., Noor M. M.Malaysian J. Biochem. Mol. Biol. 2008; 16:10–14.
(10). Giribabu N., Kumar K. E., Rekha S. S., Muniandy S., Salleh N.BMC Complement. Altern. Med. 2014; 14:291.
(11). Parhizkar S., Yusoff M. J., Dollah M. A.Adv. Pharm. Bull. 2013; 3:345–352.
(12). Idris M. H., Budin S. B., Osman M., Mohamed J.EXCLI J. 2012; 11:659–669.
(13). Fatmawati , Hidayat R.Eur. J. Pharm. Med. Res. 2016; 3:46–49.
(14). Bahrani H., Mohamad J., Paydar M. J., Rothan H. A.Curr. Alzheimer Res. 2014; 11:206–214.
(15). Hara H., Ise Y., Morimoto N., Shimazawa M., Ichihashi K., Ohyama M., Iinuma M.Biosci. Biotechnol. Biochem. 2008; 72:335–345.
(16). Kakino M., Tazawa S., Maruyama H., Tsuruma K., Araki Y., Shimazawa M., Hara H.BMC Complement. Altern. Med. 2010; 10:68.
(17). Zhou M., Wang H., Suolangjiba Kou J., Yu B. J.Ethnopharmacol. 2008; 117:345–350.
(18). Dash M., Patra J. K., Panda P. P. Afr. J.Biotechnol. 2008; 7:3531–3534.
(19). Begum Y.Pharma Tutor. 2016; 4:47–50.
(20). Pranakhon R., Pannangpetch P., Aromdee C. Songklanakarin. J.Sci. Technol. 2011; 33:405–410.
(21). Ukwuani A. N., Abubakar M. G., Hassan S. W., Agaie B. M.Int. J. Pharm. Sci. Drug Res. 2012; 4:245–249.
(22). Ali Khairullah Z., Hazilawati H., Hutheyfa S., Mohd Rosly S., Sithambaram S., Hemn Hasan O.Asian J. Pharm. Clin. Res. 2015; 8:400–408.
(23). Anisuzzaman A. S. M., Sugimoto N., Sadik G., Gafur M. A. Pak. J.Biol. Sci. 2001; 4:1012–1015.
(24). Kazmi I., Afzal M., Rahman M., Gupta G., Anwar F. Asian Pac. J.Trop. Dis. 2012; 2:S841–S845.
(25). Carro-Juárez M., Franco M. Á., Rodriguez-Peña Mde. L. J.Evid. Based Complement. Altern. Med. 2014; 19:43–50.
(26). Wil N. N. A. N., Omar N. M., Ibrahim N. A., Tajuddin S. N. J.Chem. Pharm. Res. 2014; 6:688–693.
(27). Ghan S. Y., Chin J. H., Thoo Y. Y., Yim H. S., Ho C. W.Int. J. Pharm. Sci. Res. 2016; 7:1456–1461.

(28). Veeresh Kumar P., Gupta V. R. M. J.Phytopharmacol. 2017; 6:178–182.
(29). OECD/OCDE, OECD Guidelines for the testing of chemicals 407. 2008.
(30). Erhirhie E. O., Ekene N. E., Ajaghaku D. L. J.Nat. Sci. Res. 2014; 4:100–106.
(31). Ali R., Ali R., Jaimini A., Nishad D. K., Mittal G., Chaurasia O. P., Kumar R., Bhatnagar A., Singh S. B.Indian J. Pharmacol. 2012; 44:504–508.
(32). Arsad S. S., Esa N. M., Hamzah H., Othman F. J.Med. Plant Res. 2013; 7:3030–3040.
(33). Aniagu S. O., Nwinyi F. C., Akumka D. D., Ajoku G. A., Dzarma S., Izebe K. S., Ditse M., Nwaneri P. E. C., Wambebe C., Gamaniel K. Afr. J.Biotechnol. 2005; 4:72–78.
(34). Ilgın S., Aydoğan-Kılıç G., Baysal M., Kılıç V, Ardıç M., Uçarcan Ş., Atlı Ö.Oxid. Med. Cell. Longev. 2018; 2018:7196142.
(35). Sönmez M., Türk G., Yüce A.Theriogenology. 2005; 63:2063–2072.
(36). El-Kashoury A. A., Salama A. F., Selim A. I., Mohamed R. A.Life Sci. J. 2010; 7:5–19.
(37). Akunna G. G., Saalu L. C., Ogunlade B., Ojewale A. O., Enye L. A. Am. J.Res. Commun. 2013; 1:123–142.
(38). Sakr S. A., Zowail M. E., Marzouk A. M.Anat. Cell. Biol. 2014; 47:171–179.
(39). Takeda N., Yoshinaga K., Furushima K., Takamune K., Li Z., Abe S. I., Aizawa S., Yamamura K.Sci. Rep. 2016; 6:27409.
(40). Lucio R. A., Tlachi-López J. L., Eguibar J. R., Ågmo A.Physiol. Behav. 2013; 110:73–79.
(41). Neergheen-Bhujun V. S.Biomed. Res. Int. 2013; 2013:804086.
(42). Pingale S. S., Ganpat M. A., Gawali S. Int. Res. J.Pharm. 2011; 2:263–266.
(43). Ellacott K. L., Morton G. J., Woods S. C., Tso P., Schwartz M. W.Cell. Metab. 2010; 12:10–17.
(44). Sattayasai J., Bantadkit J., Aromdee C., Lattmann E., Airarat W. J.Ayurveda Integr. Med. 2012; 3:175–179.
(45). Sanyal S., Maity P., Pradhan A., Bepari M., Dey S. K., Roy T., Choudhury S. T.Toxicol. Forensic Med. 2016; 1:54–64.
(46). Sharif H. B., Mukhtar M. D., Mustapha Y., Baba G., Lawal A. O.Adv. Pharm. 2015; 2015:1–9.
(47). Shahraki M. R., Shahraki S., Arab M. R., Shahrakipour M.Zahedan J. Res. Med. Sci. 2015; 17:42–46.
(48). Chen D., Bi D., Song Y. L., Tu P. F. Chin. J.Nat. Med. 2012; 10:287–291.
(49). Yang L., Qiao L., Xie D., Yuan Y., Chen N., Dai J., Guo S.Phytochemistry. 2012; 76:92–97.
(50). Peng K., Mei W. L., Zhao Y. X., Tan L. H., Wang Q. H., Dai H. F. J.Asian Nat. Prod. Res. 2011; 13:951–955.
(51). Chen H. Q., Wei J. H., Yang J. S., Zhang Z., Yang Y., Gao Z. H., Sui C., Gong B.Chem. Biodivers. 2012; 9:236–250.
(52). Khalil A. S., Rahim A. A., Taha K. K., Abdallah K. B.J Appl. Indus. Sci. 2013; 1:78–88.
(53). Sheweita S. A., Tilmisany A. M., Al-Sawaf H.Curr. Drug Metab. 2005; 6:495–501.
(54). Thakur M., Chauhan N. S., Bhargava S., Dixit V. K.Arch. Sex. Behav. 2009; 38:1009–1015.
(55). Cheah Y., Yang W.Adv. Biosci. Biotechnol. 2011; 2:182–197.
(56). Adenubi O. T., Raji Y., Awe E. O., Makinde J. M.Sci. World J. 2010; 5:1–6.
(57). Yousef M. I., Salama A. F.Food Chem. Toxicol. 2009; 47:1168–1175.
(59). Agarwal A., Sekhon L. H.Hum. Fertil. 2010; 13:217–225.
Go to : 
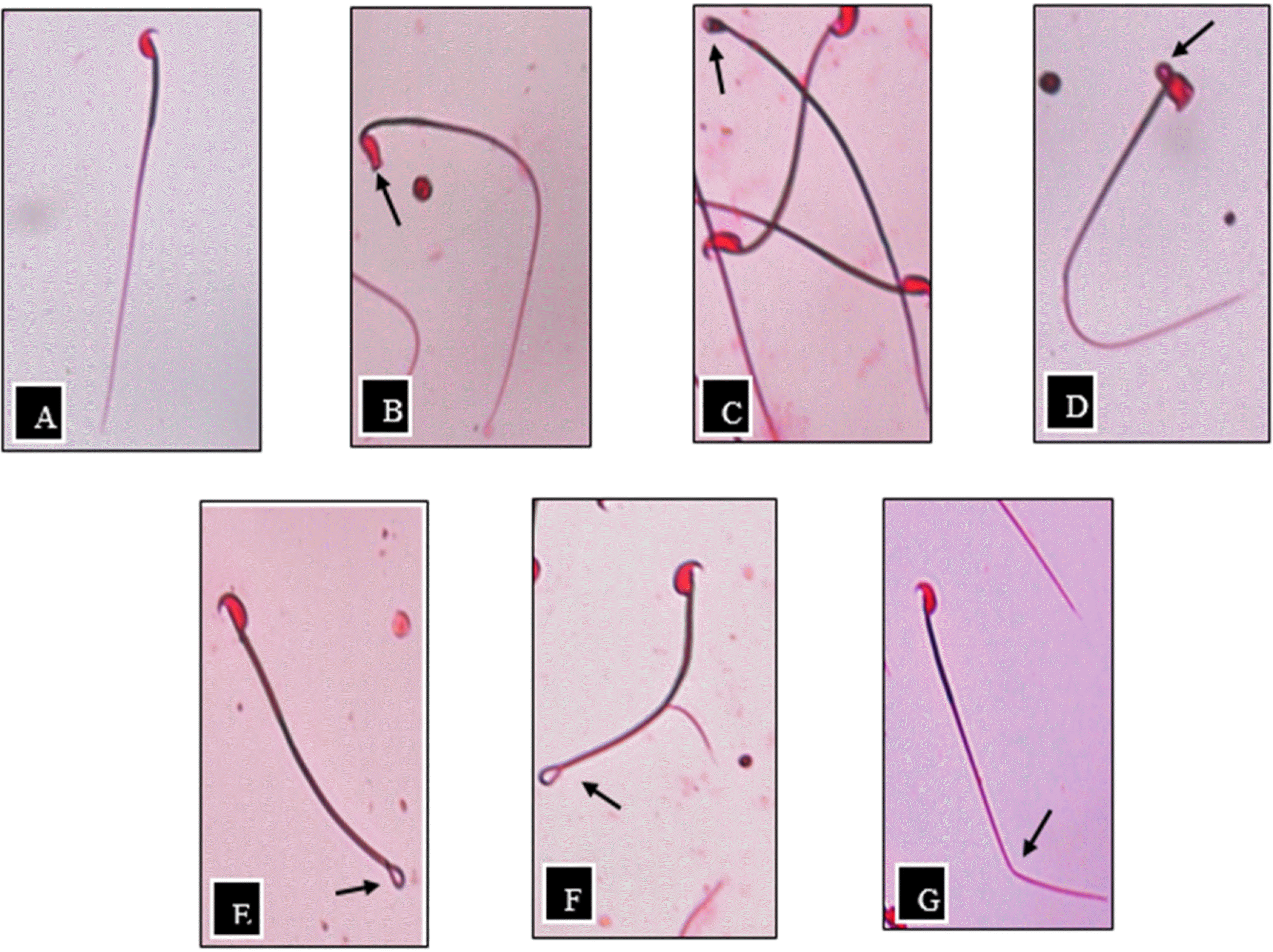 | Fig. 1.Sperm morphology of ICR male mice, as indicated by eosin staining and observed using inverted microscope (40×magnification),(A) Normal sperm, (B) No hook, (C) Pin head, (D) Bent head, (E) Coiled flagellum, (F) Hairpin loop, (G) Bent flagellum. |
Table 1.
The different doses of aqueous extract for experimental groups
Table 2.
Changes in body weight and mortality rate (%) of single dose (2000 mg/kg) of A. malaccensis leaves extract on the body weight of mice for 14 days
| Treatment Groups | Body weight in grams (mean ± SEM) | Mortality rate (%) | ||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | ||
| Control | 35.58 ± 0.50 | 35.95 ± 1.09 | 34.03 ± 1.45 | 0 |
| AEAM 2000 | 40.21 ± 1.39∗ | 38.11 ± 2.05 | 38.79 ± 1.36∗ | 0 |
Table 3.
Effects of single dose of 2000 mg/kg of A. malaccensis leaves extract on relative organ weights
Table 4.
Effects of single dose of 2000 mg/kg of A. malaccensis leaves extract on sperm parameters of mice given 14 days’ treatment
| Treatment Groups | Sperm parameters (mean ± SEM) | ||
|---|---|---|---|
| Sperm abnormality (%) | Sperm count (106/ml) | Sperm motility (%) | |
| Control | 43.57 ± 0.73 | 0.97 ± 0.17 | 49.02 ± 1.86 |
| AEAM 2000 | 44.02 ± 0.59 | 1.15 ± 0.08 | 58.38 ± 1.90∗ |
Table 5.
Changes in body weight and mortality rate (%) of mice following treatment with different doses of A. malaccensis aqueous leaf extract
| Treatment Groups | Body weight (g) (mean ± SEM) | Mortality rate (%) | |||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | ||
| Control | 33.74 ± 0.53 | 32.64 ± 0.31 | 32.85 ± 0.37 | 33.47 ± 0.64 | 0 |
| AEAM 50 | 34.34 ± 0.21 | 32.37 ± 0.63 | 31.77 ± 0.71 | 32.42 ± 0.78 | 0 |
| AEAM 100 | 35.40 ± 0.59 | 34.11 ± 1.09 | 34.16 ± 1.24 | 34.96 ± 0.99 | 0 |
| AEAM 150 | 38.18 ± 0.62∗ | 34.18 ± 1.53 | 35.02 ± 1.46 | 35.17 ± 1.65 | 0 |
| AEAM 200 | 34.61 ± 0.64 | 33.62 ± 0.60 | 31.67 ± 0.21 | 31.79 ± 0.34 | 0 |
| AEAM 500 | 39.28 ± 0.96∗ | 37.40 ± 1.17∗ | 35.45 ± 0.68 | 35.66 ± 0.82 | 0 |
| AEAM 1000 | 37.09 ± 0.40∗ | 33.16 ± 1.39 | 33.15 ± 1.13 | 32.71 ± 0.89 | 20 |
Table 6.
Effects of repeated doses of A. malacensis leaves extract on the relative organ weights of mice given 21 days’ treatment.
Table 7.
Effects of repeated doses of A. malacensis leaves extract on sperm parameters of mice given 21 days’ treatment
| Treatment Groups | Sperm parameters (mean ± SEM) | ||
|---|---|---|---|
| Sperm abnormality (%) | Sperm count (106/ml) | Sperm motility (%) | |
| Control | 41.91 ± 1.34 | 1.08 ± 0.17 | 49.23±1.41 |
| AEAM 50 | 40.33 ± 1.24 | 0.91 ± 0.22 | 50.55 ± 1.67 |
| AEAM 100 | 15.70 ± 1.33∗ | 1.36 ± 0.28 | 64.92 ± 1.57∗ |
| AEAM 150 | 26.52 ± 0.89∗ | 1.41 ± 0.12 | 63.57 ± 1.10∗ |
| AEAM 200 | 27.91 ± 0.90∗ | 0.94 ± 0.13 | 57.69 ± 0.78∗ |
| AEAM 500 | 30.97 ± 0.76∗ | 0.86 ± 0.13 | 50.80 ± 0.85 |
| AEAM 1000 | 34.66 ± 0.81∗ | 1.06 ± 0.18 | 56.11 ± 1.71∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download