Abstract
Ischemia/reperfusion-induced myocardial injury is the main cause of acute myocardial infarction. Dendropanax morbifera Léveille has been used in traditional medicines for the treatment of various diseases such as headache, infectious diseases, and general debility. However, the effect of extract from D. morbifera (EDM) on myocardial ischemic injury is still unknown. In this study, the effects of EDM on neonatal rat cardiomyocytes with hypoxia/reoxygenation (H/R) injury were investigated. The viability of cardiomyocytes with H (30 min)/R (1 h) decreased; however, treatment with EDM significantly inhibited H/R injury-induced cardiomyocyte death. Further, we observed that reactive oxygen species (ROS) generation and intracellular calcium concentration (Ca2+i) were significantly reduced in EDM-treated cardiomyocytes compared with that in H/R-injured positive control. In addition, western blotting results showed that EDM attenuated abnormal changes of RyR2 and SERCA2a genes in hypoxic cardiomyocytes. These results suggest that EDM ameliorates ROS generation and Ca2+i homeostasis to prevent dysregulation of calcium regulatory proteins in the heart, thereby exerting cardioprotective effects and reducing hypoxia-induced cardiomyocyte damage, which verifies the potential use of EDM as a new therapeutic agent for the treatment of myocardial ischemic injury.
Go to : 
REFERENCES
(1). Hausenloy D. J., Yellon D. M. J.Clin. Invest. 2013; 123:92–100.
(2). Cadenas S., Aragonés J., Landázuri M. O.Cardiovasc. Res. 2010; 88:219–228.
(3). Sabharwal S. S., Schumacker P. T.Nat. Rev. Cancer. 2014; 14:709–721.
(4). Sugamura K., Keaney J. F.Jr. Free Radic. Biol. Med. 2011; 51:978–992.
(5). Viña J., Borras C., Abdelaziz K. M., Garcia-Valles R., Gomez-Cabrera M. C.Antioxid. Redox Signal. 2013; 19:779–787.
(6). Duchen M. R., Verkhratsky A., Muallem S.Cell Calcium. 2008; 44:1–5.
(7). Yu H. Y., Kim K. S., Lee Y. C., Moon H. I., Lee J. H.Evid. Based Complement. Alternat. Med. 2012; 2012:637512.
(8). Park B. Y., Min B. S., Oh S. R., Kim J. H., Kim T. J., Kim D. H., Bae K. H., Lee H. K. J.Ethnopharmacol. 2004; 90:403–408.
(9). Moon H. I.Hum. Exp. Toxicol. 2011; 30:870–875.
(10). Chung I. M., Kim M. Y., Park W. H., Moon H. I.Pharmazie. 2009; 64:547–549.
(11). Chung I. M., Kim M. Y., Park S. D., Park W. H., Moon H. I.Phytother. Res. 2009; 23:1634–1637.
(12). Chung I. M., Song H. K., Kim S. J., Moon H. I.Phytother. Res. 2011; 25:784–786.
(13). Akram M., Kim K. S., Kim E. S., Syed A. S., Kim C. Y., Lee J. S., Bae O. N.Biol. Pharm. Bull. 2016; 39:728–736.
(14). Yu H. Y., Jin C. Y., Kim K. S., Lee Y. C., Park S. H., Kim G. Y., Kim W. J., Moon H. I., Choi Y. H., Lee J. H. J.Agric. Food Chem. 2012; 60:5400–5406.
(15). Lee J. W., Kim K. S., An H. K., Kim C. H., Moon H. I., Lee Y. C.PLoS One. 2013; 8:e83611.
(16). Jin C. Y., Yu H. Y., Park C., Han M. H., Hong S. H., Kim K. S., Lee Y. C., Chang Y. C., Cheong J., Moon S. K., Kim G. Y., Moon H. I., Kim W. J., Lee J. H., Choi Y. H.Int. J. Oncol. 2013; 43:1943–1950.
(17). Vandergriff A. C., Hensley M. T., Cheng K. J.Vis. Exp. 2015; 98:e52726.
(18). Buja L. M., Entman M. L.Circulation. 1998; 98:1355–1357.
(18). Buja L. M., Entman M. L.Circulation. 1998; 98:1355–1357.
(19). Griendling K. K., FitzGerald G. A.Circulation. 2003; 108:2034–2040.
(20). Schriewer J. M., Peek C. B., Bass J., Schumacker P. T. J.Am. Heart Assoc. 2013; 2:e000159.
(21). Barry W. H., Bridge J. H.Circulation. 1993; 87:1806–1815.
(22). Choi K. M., Zhong Y., Hoit B. D., Grupp I. L., Hahn H., Dilly K. W., Guatimosim S., Lederer W. J., Matlib M. A. Am. J.Physiol. Heart Circ. Physiol. 2002; 283:H1398–H1408.
(23). Boudina S., Abel E. D.Circulation. 2007; 115:3213–3223.
(24). Trost S. U., Belke D. D., Bluhm W. F., Meyer M., Swanson E., Dillmann W. H.Diabetes. 2002; 51:1166–1171.
(25). Kain V., Kumar S., Sitasawad S. L.Cardiovasc. Diabetol. 2011; 10:97.
(26). Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O'Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E.Cell. 2008; 133:462–474.
(27). Li Y. H., Chung H. C., Liu S. L., Chao T. H., Chen J. C.Int. Heart J. 2009; 50:207–220.
(28). Kim W., Kim D. W., Yoo D. Y., Jung H. Y., Nam S. M., Kim J. W., Hong S. M., Kim D. W., Choi J. H., Moon S. M., Yoon Y. S., Hwang I. K.BMC. Complement. Altern. Med. 2014; 14:428.
(29). Lee J. W., Park C., Han M. H., Hong S. H., Lee T. K., Lee S. H., Kim G. Y., Choi Y. H.Oncol. Rep. 2013; 30:1231–1238.
Go to : 
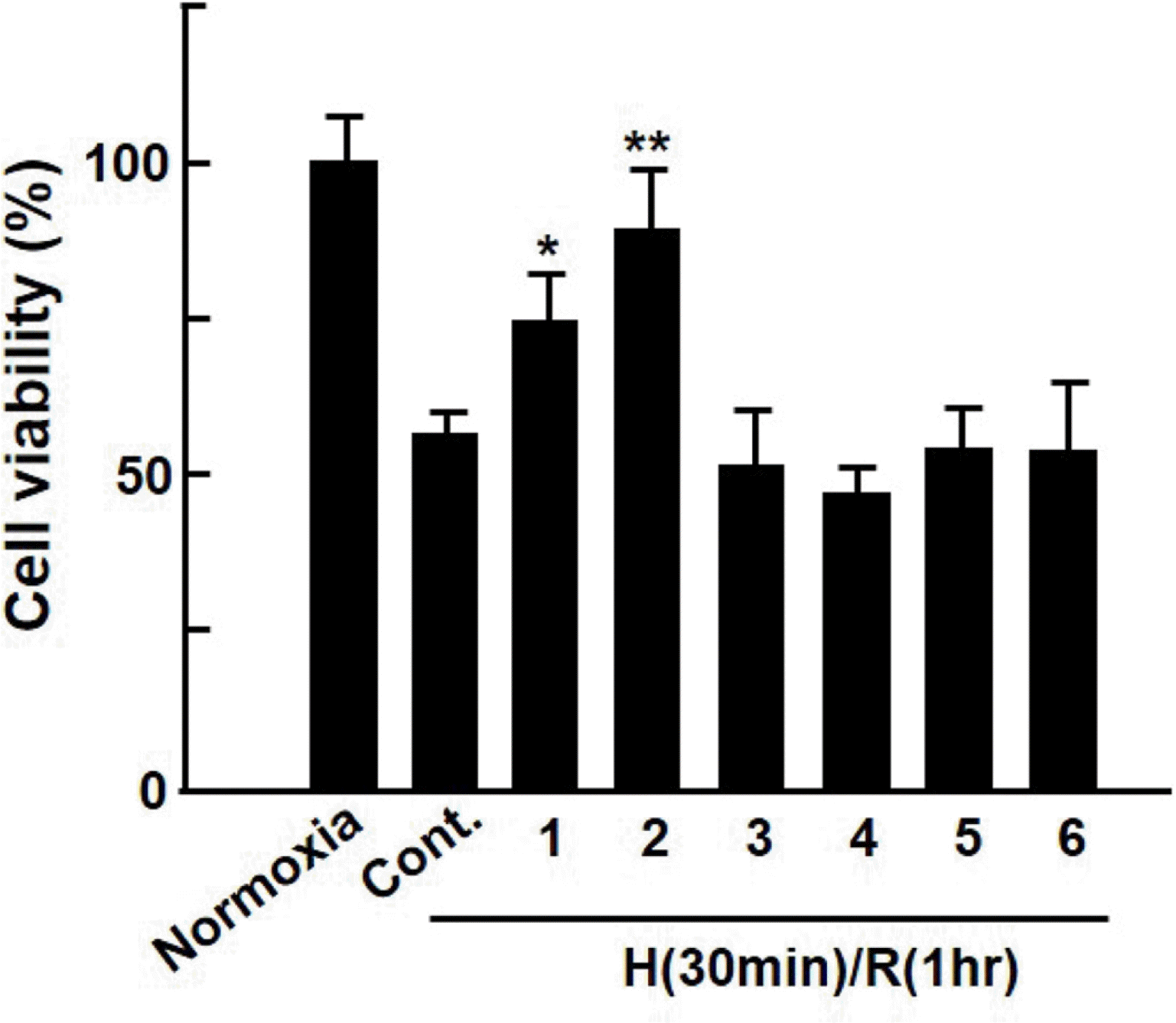 | Fig. 1.Protective effect of extract from Dendropanax morbifera on hypoxia/reoxygenation-injured cardiomyocytes. Cardiomyocytes were subjected to hypoxia (30 min)-reoxygenation (1 h) after 1 h of Dendropanax morbifera extract (EDM) treatment. The cell viability was evaluated after 24 h using MTT assay. Values are presented as mean ± S.D. ∗P < 0.05 or ∗∗P < 0.01 vs. control. |
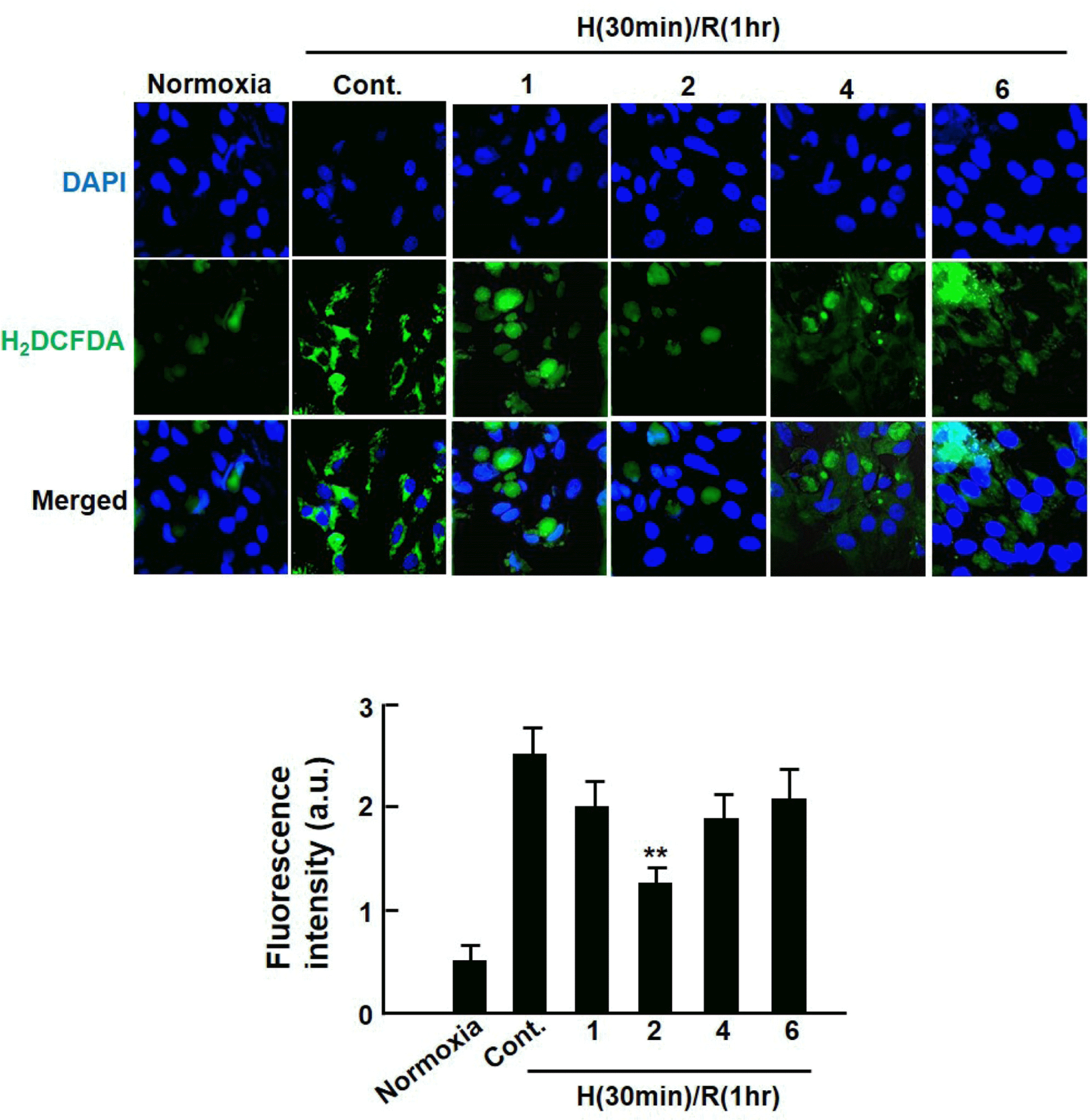 | Fig. 2.Inhibitory effect of extract from Dendropanax morbifera on intracellular ROS generation in hypoxia/reoxygenation-injured cardiomyocytes. Cardiomyocytes were subjected to hypoxia (30 min)-reoxygenation (1 h) after 1 h of Dendropanax morbifera extract (EDM) treatment. Then, fluorescent H2DCF-DA images were taken using a confocal laser microscope (Olympus, Japan) at excitation and emission wavelengths of 488 and 520 nm, respectively (fluorescent images were magnified: ×400). The fluorescence intensity of an equivalent field size (2.5 × 2.5 mm) was measured using the Image J quantification software. Values are presented as mean ± S.D.∗∗ P < 0.01 vs. control. |
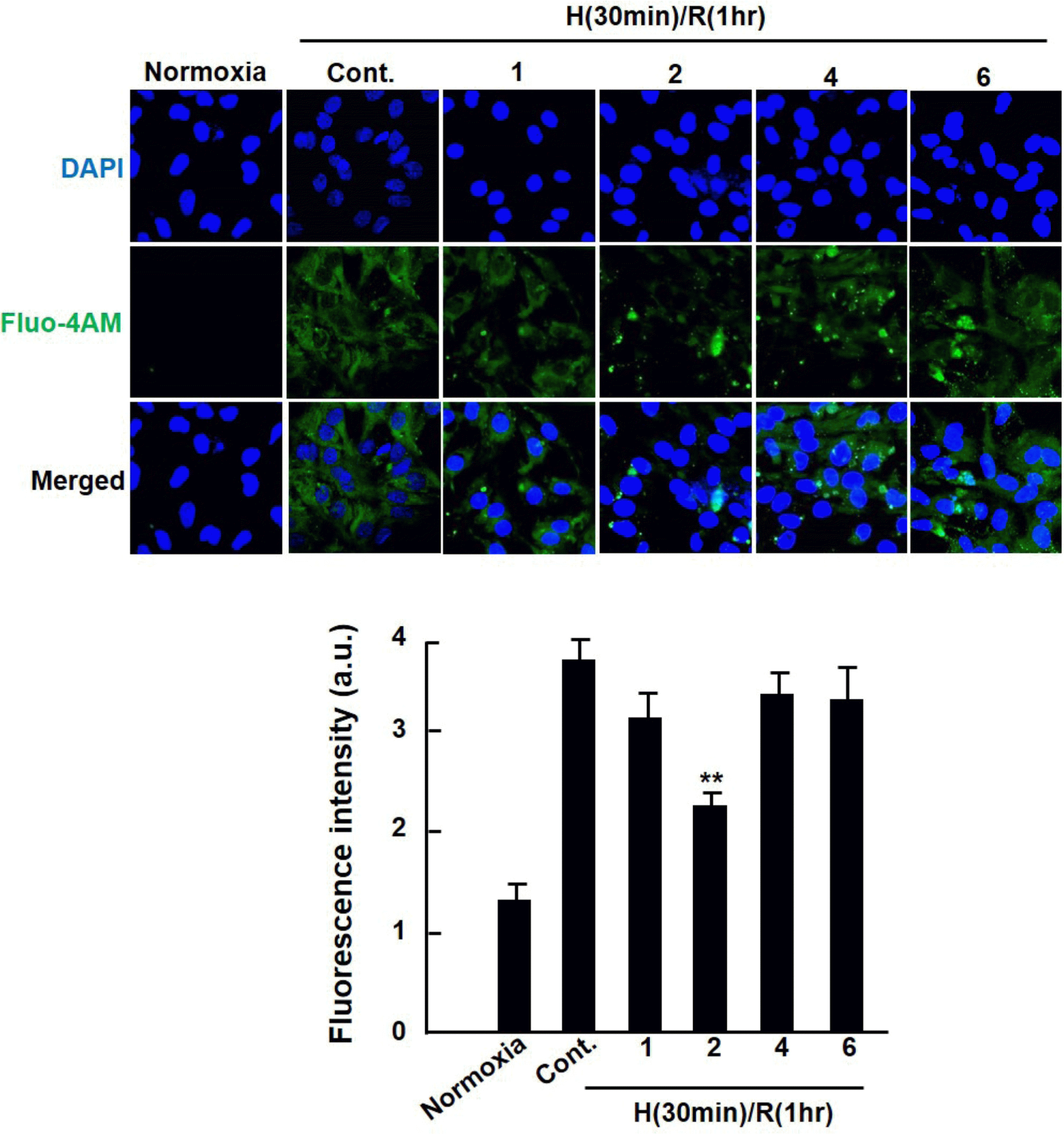 | Fig. 3.Effect of extract from Dendropanax morbifera on intracellular calcium perturbation in hypoxia/reoxygenation-injured cardiomyocytes. Cardiomyocytes were subjected to hypoxia (30 min)-reoxygenation (1 h) after 1 h of Dendropanax morbifera extract (EDM) treatment. Fluorescent Fluo-4am images were taken by a confocal laser microscope (Olympus, Japan). Values are presented as mean ± S.D. ∗P < 0.05 vs. control. |
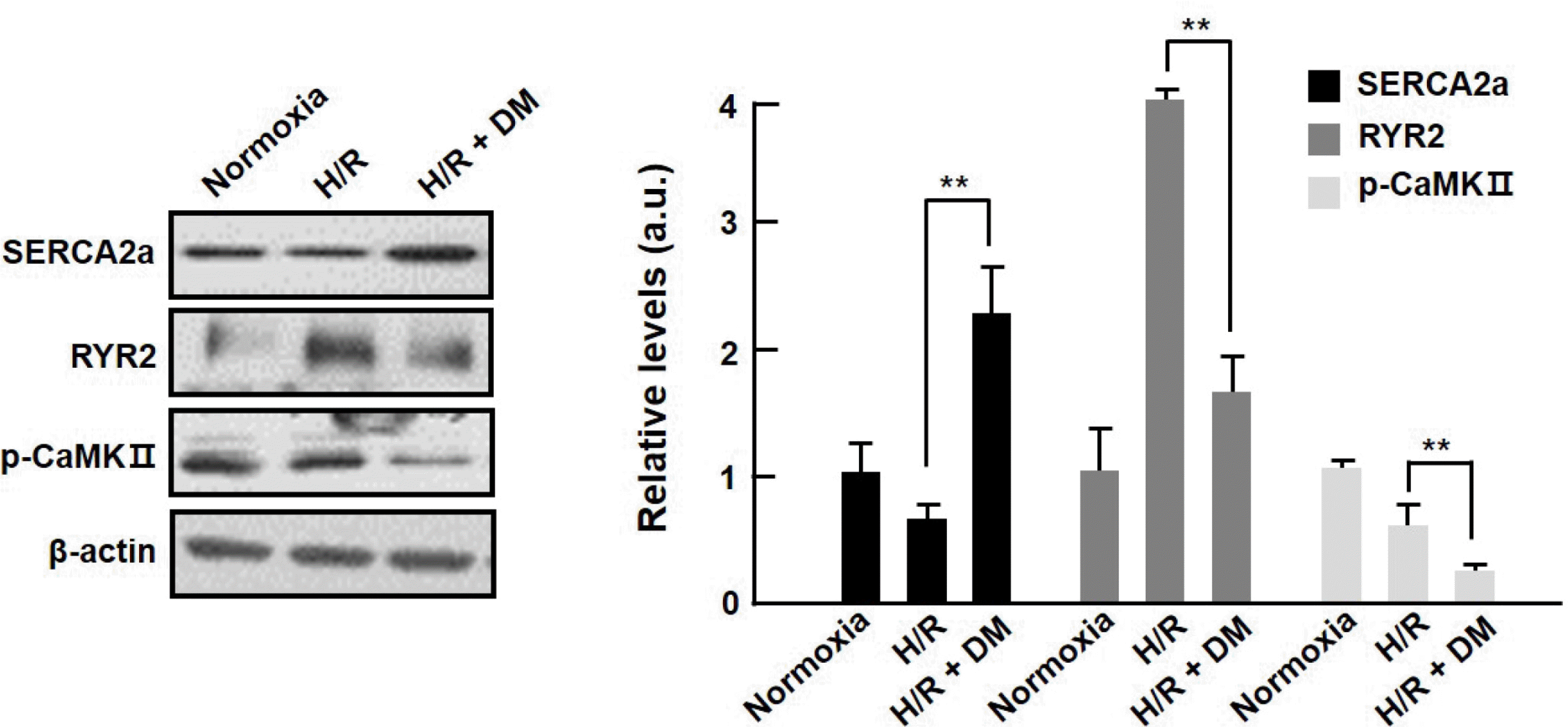 | Fig. 4.Altered expression levels of calcium homeostasis-related proteins by extract from Dendropanax morbifera in hypoxia/ reoxygenation-injured cardiomyocytes. Cardiomyocytes were subjected to hypoxia (30 min)-reoxygenation (1 h) after 1 h treatment with #2 of Dendropanax morbifera extract (EDM). The levels of SERCA2a, p-CaMKII, and RyR2 were evaluated by western blotting. Values are presented as mean ± S.D. ∗∗P < 0.01 vs. control. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download