INTRODUCTION
MATERIALS AND METHODS
Subjects
Mice
Induction of AD
Quantitative real-time polymerase chain reaction (PCR)
Table 1
Sequences of the primers used for quantitative real-time polymerase chain reaction

Enzyme-linked immunosorbent assay (ELISA)
Hematoxylin and eosin (H&E) staining
Electron microscopy
Flow cytometry
Statistical analysis
RESULTS
ALCAM levels are altered in serum from human AD and OVA-induced AD model
Table 2
Characteristics of the study population

 | Fig. 1ALCAM expression in pediatric AD patients and OVA-induced AD mice. (A-C) ALCAM level was measured by ELISA in serum samples from healthy controls (n = 44) and AD patients (n = 114). Pirate plots show the distribution of ALCAM levels for each group; horizontal lines represent means and boxes represent 95% confidence intervals. (A) ALCAM level in human serum. (B) Increase in serum ALCAM level as a function of AD severity increases. (C) Correlation between serum ALCAM level and AD SCORAD index. (D) Serum ALCAM abundance was assessed by ELISA.The data represent mean ± standard error of the mean.
ALCAM, activated leukocyte cell adhesion molecule; AD, atopic dermatitis; OVA, ovalbumin; PBS, phosphate-buffered saline; SCORAD, SCORing atopic dermatitis; ELISA, enzyme-linked immunosorbent assay.
*P < 0.05; †P < 0.001 by t-test (n = 5–10 mice/group).
|
ALCAM deficiency alleviates skin barrier disturbance
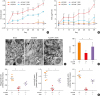 | Fig. 2ALCAM attenuates skin barrier disruption induced by epicutaneous OVA sensitization. (A) Time course of clinical score and (B) TEWL. (C) Electron micrographs of osmium tetroxide-postfixed skins show LBs in SG (×50,000, Scale bar = 0.5 μm; Insert C). (Left) Normal shaped LBs are present in sham mouse epidermis. (Middle) WT/OVA mouse epidermis shows severely abnormal LBs, (Right) Whereas LBs in ALCAM−/− mice display less abnormality (×120,000, Scale bar = 0.2 μm). (D) Bar graph represents the number of LBs in SG. (E) The mRNA expression of skin barrier genes (filaggrin, loricrin and involucrin).The data represent mean ± standard error of the mean.
ALCAM, activated leukocyte cell adhesion molecule; TEWL, transepidermal water loss; OVA, ovalbumin; PBS, phosphate-buffered saline; NS, not significant; WT, wild-type; LB, lamellar body; mRNA, messenger RNA; SG, stratum granulosum.
*P < 0.05 (WT/PBS vs. WT/OVA); †P < 0.05 (ALCAM−/−/PBS vs. ALCAM−/−/OVA); ‡P < 0.05 (WT/OVA vs. ALCAM−/−/OVA); §P < 0.05; ∥P < 0.01; ¶P < 0.001 (n = 5–7 mice/group).
|
ALCAM deficiency suppresses Th-2 inflammation
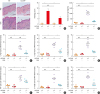 | Fig. 3ALCAM deficiency attenuates T helper-dominant skin inflammation induced by epicutaneous OVA sensitization. (A) Mouse skin biopsy specimen stained with hematoxylin and eosin (×200, Scale bar = 50 μm) and scored according to histological features. (B) Serum levels of total IgE measured by enzyme-linked immunosorbent assay. (C-H) Skin mRNA levels were assessed by quantitative real-time polymerase chain reaction; (C) IL-4, (D) IL-5, (E) IL-13, (F) IFN-γ, (G) IL-17A and (H) IL-22.The data represent mean ± standard error of the mean.
ALCAM, activated leukocyte cell adhesion molecule; NS, not significant; OVA, ovalbumin; PBS, phosphate-buffered saline; WT, wild-type; IgE, immunoglobulin E; IL, interleukin; IFN, interferon; mRNA, messenger RNA.
*P < 0.05; †P < 0.01; ‡P < 0.001 (n = 5–7 mice/group).
|
ALCAM deficiency suppresses CD4 T cell activation
 | Fig. 4ALCAM deficiency suppresses CD4+ T cell activation. Cells from the (A and B) skin and (C and D) skin draining -LN(dLN)s were harvested and stained for CD3, CD4, CD44 and CD62L. Cells were gated on CD3+CD4+ cells and then on CD44 and CD62L. Naïve cells are identified as CD44loCD62Lhi and effector cells as CD4hiCD62Llo. Representative dot plots are presented. Graphs represent percentage of (B) CD3+CD4+CD44loCD62Lhi in skin and (D) CD3+CD4+CD4hiCD62Llo in dLNs.The data represent mean ± standard error of the mean.
ALCAM, activated leukocyte cell adhesion molecule; WT, wild-type; NS, not significant; OVA, ovalbumin; dLN, skin-draining lymph node.
*P < 0.05; †P < 0.01; ‡P < 0.001(n = 5–7 mice/group).
|
ALCAM deficiency suppresses skin inflammation and barrier disruption in the OXA- induced AD-like model
 | Fig. 5ALCAM deficiency attenuates murine AD-like skin lesion induced by repeated OXA application. (A-C) AD severity was assessed by (A) clinical score, (B) TEWL and (C) ear thickness. (D) Mouse ear skin biopsy specimen stained with hematoxylin and eosin (×100, bar = 200 μm) and (E) scored according to histological features. (F) Total IgE level in serum, as determined by enzyme-linked immunosorbent assay. The mRNA expression of (G) IL-4 and IL-13 in skin lesions.Data represent mean ± standard error of the mean.
ALCAM, activated leukocyte cell adhesion molecule; OXA, oxazolone; AD, atopic dermatitis; mRNA, messenger RNA; NS, not significant; WT, wild-type; TEWL, transepidermal water loss; IgE, immunoglobulin E; IL, interleukin, PBS, phosphate-buffered saline.
*P < 0.05 (WT/PBS vs. WT/OXA); †P < 0.05 (ALCAM−/−/PBS vs. ALCAM−/−/OXA); ‡P < 0.05 (WT/OXA vs. ALCAM−/−/OXA); §P < 0.05; ∥P < 0.01; ¶P < 0.001 (n = 4–6 mice/group).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download