Abstract
In this study, we report arterial spin labelling perfusion, proton MR spectroscopy and susceptibility-weighted MR findings of acute necrotizing encephalopathy in a child with rotavirus infection.
Acute necrotizing encephalopathy (ANE) is an extremely rare disease that was first proposed by Mizuguchi et al. (1) in 1995. ANE mostly develops secondary to viral infections, such as influenza virus A, B and H1N1, herpes simplex virus (HSV) and human herpes virus-6 (HHV-6) (12). There have been several reports on conventional MR imaging findings of ANE. However, findings on advanced MR imaging techniques, such as arterial spin labelling (ASL) perfusion MR, have been rarely described. In this study, we report ASL perfusion MR, proton MR spectroscopy (MRS) and susceptibility-weighted imaging (SWI) findings of ANE in a child patient.
Previously, a healthy 18-month-old girl was admitted to our emergency department with a sudden generalized tonic-clonic (GTC) seizure. She had a 3 day history of fever and diarrhea. An hour after admission, she experienced two episodes of the GTC type seizure with decreased consciousness. The following day, her motor functions of the bilateral upper and lower extremities fell to grade 3/3.
On the first blood serum test, C-reactive protein (CRP), glucose, uric acid, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and ammonia were reported to be 47.8 mg/L (normal range, 0-5 mg/L), 413 mg/dL (70-110 mg/dL), 13.7 mg/dL (2.4-5.7 mg/dL), 267 IU/L (35-130 IU/L), 57 IU/L (0-37 IU/L), 55 IU/L (0-41 IU/L), 356 IU/L (135-225 IU/L) and 109 µg/dL (19-87 µg/dL), respectively. The rotavirus test was positive on her stool examination. Complete blood count (CBC), urine test, cerebrospinal fluid (CSF) analysis, pyruvic acid, amino acids and insulin were within normal limits.
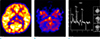
On the second day of admission, a brain MR examination was performed in a 3T machine (MAGNETUM Skyra, Siemens, Germany). The T2-weighted and fluid-attenuated inversion recovery (FLAIR) images showed symmetrical hyper intense lesions in the cerebellum, pons, midbrain, thalami, basal ganglia and cerebral white matter. The lesions showed restricted diffusion on the diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map. On the SWI, there were multiple hemorrhagic foci in the thalamic lesions (Fig. 1). An ASL perfusion imaging was obtained by using a 2D pseudo-continuous ASL (pCASL) pulse sequence with the following scan parameters: repetition time (TR)/echo time (TE) 4000 ms/12 ms, label duration 1650 ms, post label delay 1600 ms, label distance 98 mm and a number of dynamic scans 25. The relative cerebral blood flow (rCBF) map images showed a decreased perfusion in the lesions of the thalami and cerebellum (Fig. 2). A proton MRS was performed by using a 2D multi-voxel point-resolved spectroscopy sequence (TR/TE 1520 ms/135 ms, voxel size 7.5 × 7.5 × 10 mm, NEX 4, scan duration 3 minutes 57 seconds). The volumes of interest (VOIs) were centered at the level of the thalamus. The proton MRS showed an abnormal lactate peak in the thalamic lesions (Fig. 2).
The patient received supportive care and began to receive cefotaxime, acyclovir, azithromycin hydrate, methylprednisolone and intravenous immunoglobulin (IVIG). On the 7th hospital day, the motor power of her upper and lower limbs improved from grade 3/3 to 4/4 and her consciousness was fully recovered.
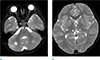
The follow-up MR examination was obtained 3 years later. From the results, there were focal tissue losses in both the cerebellum, right basal ganglia and both thalami (Fig. 3). Even though there were focal tissue losses in the imaging findings, she was not delayed in the development compared to the same age group.
ANE predominantly affects healthy infants and young children. Even though the influenza virus and HHV-6 are the common pathogens of the preceding infection associated with ANE, as in our patient, the rotavirus can also be associated with ANE (1, 3). Hoshino et al. (3) reported that ANE was associated with rotavirus infection in one of the 39 patients (2.4%).
Mizuguchi proposed a diagnostic criteria for ANE with clinical and specific radiologic findings (2). The main radiologic features of the ANE are the multifocal and bilateral involvement of the thalamus, tegmentum of the upper brain stem, cerebral white matter and cerebellum on CT and MR (12456). The lesions show strong diffusion restriction on the DWI (45). Our patient also showed similar findings, however, the lesions were very extensive. Hemorrhage is commonly associated with ANE, especially in the lesions of the thalamus (5). In our patient, the SWI showed multiple hemorrhagic foci in both thalami, which was not detected on the T1, T2 and DWIs. The SWI is the most sensitive MR sequence for the detection of hemorrhage and it has been replacing the conventional gradient echo T2*-weighted image. In our patient, the proton MRS with intermediate TE showed an inverted lactate doublet at 1.33 ppm. According to the previous reports, the proton MRS can detect an abnormal lactate and lipids peaks as well as an increase in the glutamate/glutamine complex in patients with ANE (7).
To our knowledge, this is the first report on perfusion MR for the evaluation of ANE. Our patient showed decreased perfusion in both thalami and cerebellum on the rCBF maps of the ASL perfusion MR. A decreased CBF in the bilateral thalamic regions has also been reported as the ASL finding of the seizure at the interictal phase. In our case, we cannot completely exclude the effect of the seizures. However, the ASL perfusion MR was performed within 24 hours after the last seizure, the degree of the CBF reduction was very severe, and the CBF of both cerebellar lesions was also decreased. Taking into consideration these factors, we think that the possibility of seizure effects could be ruled out. The ASL technique is a non-invasive tool that is used to measure the CBF without administering a radiopharmaceutical or contrast agent and can be repeatedly used. There have been several case reports of single photon emission tomography (SPECT) in patients with ANE. Most patients showed hypoperfusion. However, they were in the chronic stage (8). Hayakawa et al. (9) reported an early state ANE that showed hyper perfusion bilaterally in the thalami. We cannot explain with certainty why the perfusion status between our patient and theirs' are different. However, it might be caused by the differences in the patient, imaging modality and timing of imaging acquisition. They obtained SPECT images on the 12th day of admission. Further studies with a large number of patients are needed in order to identify more accurate results.
The disease could have clinical consequences, such as high grade fever, seizures and neurologic deficits with or without severe sequelae (12). Wong et al. (6) proposed an MR-based scoring system that has a positive correlation with the clinical outcomes in ANE patients. According to the Wong's report, characteristic factors including the involvement of the brain stem and cerebral white matter and presence of hemorrhage and cavitation showed poor prognosis. The patient's outcome was not poor, even though high scores were obtained when applying the MR-based scoring system in our patient because of the brain stem, basal ganglia and cerebral white matter involvement, hemorrhage and neurologic deficits.
There have been no exact recommended treatment guidelines for the ANE. However, in some patients corticosteroids have improved clinical conditions and prognosis in the acute phase. In addition, hypothermia, early anticytokine therapy, immunoglobulin and plasmapheresis have been suggested as treatments in some cases of ANE (1011). Although the ANE is a destructive and progressive disease that can leave neurologic sequelae, we believe our patient had a good prognosis since she was initially treated with steroids, IVIG, antiviral agents and empirical antibiotics.
In summary, ANE is a rare disease entity that has typical radiologic features, multifocal and bilateral involvement of the thalamus, cerebral white matter, brain stem and cerebellum. Hemorrhage can be commonly associated in the lesions of the ANE and it can be sensitively detected on the SWI. The proton MRS and ASL perfusion images showed an abnormal lactate peak and decreased CBF in the lesions, respectively. However, as it is a result of one patient, further studies are needed.
Figures and Tables
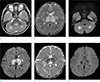 | Fig. 1An 18-month-old girl with a sudden generalized tonic-clonic (GTC) seizure. (a, b) Axial T2 and (c, d) diffusion-weighted images show symmetric hyper intense lesions in the bilateral cerebellum, thalami and basal ganglia. (e) On ADC map image, the lesions revealed restrict diffusion except for the peripheral portion. (f) There are multiple hemorrhagic foci in the bilateral thalami on the susceptibility-weighted image. |
References
1. Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995; 58:555–561.

2. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997; 19:81–92.

3. Hoshino A, Saitoh M, Oka A, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012; 34:337–343.

4. Albayram S, Bilgi Z, Selcuk H, et al. Diffusion-weighted MR imaging findings of acute necrotizing encephalopathy. AJNR Am J Neuroradiol. 2004; 25:792–797.
5. Kim JH, Kim IO, Lim MK, et al. Acute necrotizing encephalopathy in Korean infants and children: imaging findings and diverse clinical outcome. Korean J Radiol. 2004; 5:171–177.

6. Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol. 2006; 27:1919–1923.
7. Goo HW, Choi CG, Yoon CH, Ko TS. Acute necrotizing encephalopathy: diffusion MR imaging and localized proton MR spectroscopic findings in two infants. Korean J Radiol. 2003; 4:61–65.

8. Oki J, Yoshida H, Tokumitsu A, et al. Serial neuroimages of acute necrotizing encephalopathy associated with human herpesvirus 6 infection. Brain Dev. 1995; 17:356–359.

9. Hayakawa J, Fujino O, Murakami M, Fukunaga Y. Unusual findings in single-photon emission computed tomography in a 1-year-old boy with acute necrotizing encephalopathy. Pediatr Int. 2007; 49:94–96.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download