Abstract
Hyperglycemia-induced hemichorea (HGHC) is a rare but characteristic hyperkinetic movement disorder involving limbs on one side of the body. In a 75-year-old woman with a left-sided HGHC, conventional brain MR imaging showed very subtle T1-hyperintensity and unique gadolinium enhancement in the basal ganglia contralateral to movements. Multi-parametric MRI was acquired using pulse sequence with quantification of relaxation times and proton density by multi-echo acquisition. Myelin map was reconstructed based on new tissue classification modeling. In this case report of multi-parametric MRI, quantitative measurement of myelin change related to HGHC in brain structures and its possible explanations are presented. This is the first study to demonstrate myelin loss related to hyperglycemic insult in multi-parametric quantitative MR imaging.
Hyperglycemia-induced hemichorea (HGHC) is a rare but characteristic movement disorder involving limbs on one side of the body. Along with elevated blood glucose and hemoglobin A1c levels with poorly controlled diabetes, choreiform or ballistic involuntary movements and neuroimaging findings such as CT-hyperattenuation or T1-hyperintensity on the striatum contralateral to the symptom side can lead to clinical diagnosis of HGHC (123). In correlation with neuroimaging abnormalities, pathophysiological mechanism that leads to abnormal movements has been rarely described in the literature (1234).
Multi-parametric MRI is a recent technique that can quantify longitudinal T1 relaxation, transverse T2 relaxation, proton density (PD), and amplitude of local radio frequency B1 field using multi-slice, multi-echo, and multi-delay acquisition (56789). Based on these data, any contrast-weighted image with a combination of echo time (TE), repetition time (TR), and inversion time (TI) can be created and contrast-weighting can be freely adjusted. Furthermore, myelin map can be reconstructed based on new tissue classification modeling (678). Since myelin sheath coating axon is crucial for efficient signal transmission in the nervous system to increase the propagating speed of impulses along axons, measurements and monitoring of myelin content would provide important information for the diagnosis and prognosis of patients with suspected myelin degradation. However, myelin loss related to hyperglycemic insult has not been presented in patients with HGHC yet. Here, we report multi-parametric MRI of an elderly female patient with HGHC. Quantitative measurement of myelin change related to HGHC in brain structures and its possible explanations are described.
A 75-year-old female who had achieved complete resolution from large-B cell lymphoma and been stationary for a year after chemotherapy (Fig. 1) developed left side hemichorea a month after onset of hyperglycemia. Chorea was severe at the left forearm and hand, showing dystonic and flipping feature (Video 1). Left leg showed a bit external rotation and stamping like motion during walking. Her right side limb showed relatively decreased movement such as decreased arm swing.
At ten days after hemichorea occurrence, conventional and synthetic images were obtained without contrast enhancement using a 3.0 T MRI system (Architect, GE Healthcare, Milwaukee, WI, USA) implemented with a 48-channel head coil. Conventional images included T2-weighted image (T2WI), fluid attenuated inversion recovery (FLAIR), susceptibility weighted image (SWI), and arterial spin labeling cerebral blood flow (ASL CBF). Synthetic image was acquired using pulse sequence with quantification of relaxation times and proton density by multi-echo acquisition of saturation-recovery using turbo spin-echo readout (QRAPMASTER). Incidentally, a suspicious capillary telangiectasia in the lower pons with a low intensity was found on susceptibility-weighted imaging with gadolinium-enhancement at contrast enhanced T1WI. Otherwise, no signal alteration was found on FLAIR, T2WI, SWI, or diffusion-weighted image. ASL CBF map did not show significant abnormality either.
Although there was no symptom improvement, she was discharged. At three weeks after hemichorea occurrence, second MRI performed for persisting symptom at another hospital revealed parenchymal enhancement on gadolinium-enhanced T1WI in the right putamen compared to the contralateral side (Fig. 1c, d). She was referred back to our institution for further evaluation of suspected hemichorea which made the author review her medical records again (Fig. 2). At one month before hemichorea development, she complained of aggravated general weakness, dizziness, and poor oral intake. Cognitive function appeared to be decreased with non-fluent speech. In addition, she could not find her way home. Brain CT at that time showed no significant abnormality except mild dilatation on both lateral ventricles and third ventricles (Fig. 1b). Her fasting blood glucose concentration was 816 mg/dL with HbA1c of 11.0%. Normal arterial blood gas analysis revealed that she had trace levels of urine ketones. Laboratory work-up revealed that her complete blood count was within normal range. Electrolyte imbalance was noted, including the following results: sodium, 131 mEq/L; potassium, 3.3 mEq/L; and total carbon dioxide, 21.9 mmol/L. Serum glucose was 521 mg/dL. For management of hyperglycemia, short acting insulin drip was started. After ten days of treatment, her blood glucose was well-controlled by insulin and her cognitive functions were improved.
Based on the second MRI and clinical findings, HGHC was highly suspected as a diagnosis. Synthetic MRI sequence was analyzed retrospectively. Synthetic image was reconstructed into multiple contrasts including T1, T2, R1, R2, and PD using SyMRI Stand Alone software (SyMRI, Linkoping, Sweden). Myelin-water-fraction in the brain was further estimated from R1, R2, and PD values with the SyMRI software. This estimation was based on the assumption of four compartments in the brain including myelin partial volume (MYvol), cellular partial volume, free water partial volume, and excess parenchymal water partial volume (678). This model postulates that each compartment has its own R1, R2, and PD that contribute to effective R1, R2, and PD of each specific acquisition voxel. In particular, MYvol contains myelin sheaths and water trapped between them while myelin water fraction corresponds to PD in the MYvol divided by PD in cellular and free water partial volume (78). The proportion of myelin-water-fraction to actual myelin volume has been previously validated in vitro based on histopathological examination (9). When brain structures of one side were compared to those of the other side, synthetic T1-WI demonstrated very subtle asymmetric intensity on right putamen compared to left side putamen. In addition, T2 map or PD map did not show signal asymmetry (Fig. 3). No abnormal intensity lesion was demonstrated on synthetic FLAIR (TR 15,000 ms; TE 100 ms; TI 3000 ms) and double inversion recovery (DIR) (TR 15,000 ms; TE 100 ms; initial TI 470 ms; second TI 3750 ms) images.
Quantitative analysis was performed by registering synthetic multi-contrast images into Montreal Neurological Institute (MNI) brain atlas. Patient's data were then compared with those of normal controls (Fig. 4). To obtain average value of normal controls, six age- and sex- matched normal volunteers without any neurologic deficit were registered into MNI space. Values of the predetermined area in putamen, pallidum, caudate nucleus, thalamus, red nucleus, and substantia nigra were averaged.
T1 relaxation times of the patient were longer than those of normal controls in bilateral thalamus (patient vs. normal controls: right, 1710 ms vs. 1131 ms; left, 1765 ms vs. 1292 ms), caudate nucleus (right, 2766 ms vs. 1484 ms; left, 3421 ms vs. 2004 ms), and left red nucleus (1593 ms vs. 970 ms). T2 relaxation times of patient were higher than those of normal controls in bilateral thalamus (patient vs. normal controls: right, 192 ms vs. 103 ms; left, 161 ms vs. 123 ms), caudate nucleus (right, 352 ms vs. 154 ms; left, 421 ms vs. 241 ms), and left red nucleus (159 ms vs. 89 ms).
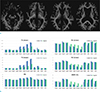
Compared to average values of normal controls, myelin-water-fractions of patient in the bilateral caudate nucleus (patient vs. normal controls: right, 3.7% vs. 12.1%; left, 1.0% vs. 7.2%), thalamus (right, 11.0% vs.18.0%; left, 9.3% vs. 16.3%), red nucleus (right, 16.2% vs. 26.3%; left, 13.4% vs. 25.0%), and substantia nigra (right, 22.0% vs. 29.0%; left, 11.2% vs. 20.7%) were markedly reduced. However, myelin-water-fractions in putamen (right, 18.6% vs. 18.6%; left, 19.1% vs. 17.1%) or pallidum (right, 27.6% vs. 24.3%; left, 27.0% vs. 25.8%) were not remarkably different from those of normal controls. On two-month follow-up MRI, myelin-water-fractions of patient in the thalamus (right, 6.8%; left, 7.8%) showed further decreases slightly while those in red nucleus (right, 24.3%; left, 24.0%) and substantia nigra (right, 25.4%; left, 19.1%) were mildly improved. However, as seen from visual assessment comparing right and left brain structures, no differences in gross values of T1, T2, R1, R2, PD, or myelin-water-fraction were found between right and left sides. 18F-fludeoxyglucose positron emission tomography (FDG-PET) performed at one month after HGHC onset showed decreased regional cerebral glucose metabolism in the right basal ganglion. Involuntary movements of the right arm were partially relieved by Haloperidol, Rivotril, and Gliatamine accompanied with strict control of blood glucose.
Signal alterations of imaging were very subtle in the contralateral putamen to the symptom side. They were not apparent upon retrospective review of initial CT or first MRI. Initial abnormality was indistinctive in brain CT at a month before HGHC occurrence. Hyperglycemia was controlled by insulin treatment for ten days. Time interval between HGHC onset and the first MRI acquisition was another ten days. Due to indistinct T1-intensity alteration, diagnosis of HGHC was delayed until second MRI acquisition with gadolinium enhancement.
Second MRI performed three weeks later demonstrated area of parenchymal enhancement in the contralateral putamen which was a helpful diagnostic clue. It was compatible to the triad of HGHC syndrome: (a) poorly controlled diabetic patients with nonketotic hyperglycemia, (b) sudden-onset hemiballism or hemichorea, and (c) contralateral striatal T1-hyperintensity or CT-hyperdensity (1234). It was further supported by the demographic feature of high incidence in Asian, elderly, and women with diabetes mellitus type II (12). The parenchymal enhancement might be understood as suggestive of blood-brain barrier (BBB) permeability. BBB destruction might be the result of hyperglycemia-mediated injury. However, she did not show any evidence of microbleeds or microhemorrhages on SWI, in discordance with previous studies insisting that petechial hemorrhage might be due to erythrocyte diapedesis (3). Interestingly, there was no diffusion restriction or any kind of edema of either cytotoxic or vasogenic origin. This allowed easy differentiation from acute infarction or ischemia. Theory of hyperviscosity was not compatible due to the lack of diffusion restriction (24).
Initial MR image was analyzed retrospectively. It was acquired using pulse sequence with QRAPMASTER and reconstructed into multiple contrast using dedicated post-processing software (5678). When brain structures of one side were compared to those of the other side, synthetic T1 map showed very subtle low values in the contralateral putamen to the side of involuntary movement while synthetic T2 map and PD map showed nearly-identical values between right and left sides.
Myelin-water-fractions in the brain were estimated from R1, R2, and PD values quantified with SyMRI software. The synthetic myelin map indicated that myelin-water-fractions decreased in affected brain structures. To the best of our knowledge, this is the first study to report quantitative myelin content change in a patient with HGHC. Interestingly, different from unilaterality of striatal high intensity lesion and presenting symptom, differences between right and left brain structures were not remarkable in myelin measurement. However, myelin-water-fractions in the caudate nucleus, thalamus, substantia nigra, and red nucleus were markedly reduced compared to average values of normal controls. This finding of bilateral myelin loss in HGHC patient has not been presented previously. Low myelin content of deep gray matters might be inferred in correlation with neurotransmitter-induced insults. Two major theories of mechanism have been suggested regarding how non-ketotic hyperglycemia induces dyskinesia. Hyperglycemia causes γ-aminobutyric acid (GABA) transmission interruption and/or dopamine upregulation at striatum (110). In non-ketotic hyperglycemia, shift to anaerobic metabolism causes brain to utilize GABA as an alternate energy substrate which causes rapid depletion of GABA and interrupts GABAnergic transmission. As GABA is the neurotransmitter responsible for both direct and indirect inhibitory pathways in the basal ganglia, imbalance between indirect excitatory and direct inhibitory pathways ultimately can lead to disinhibition of the motor thalamus and cause motor cortex to be over-excited. In a similar manner, hyperglycemia may induce upregulation of dopamine receptors or decrease dopamine catabolism in the striata, consequently increasing the excitatory effect of thalamus on cortex by dysregulating direct and indirect pathways. Further investigations coupling this neurotransmitter and myelin reduction related to its insult might be necessary in future studies.
On follow up imaging, loss of myelin-water-fraction was slightly restored in substantia nigra and red nucleus in accordance with mild symptomatic improvement. On the other hand, it seemed that myelin-water-fractions were further decreased in the thalamus. These differential myelin content changes related to functional recovery need to be investigated in a future multicenter clinical registry.
In this patient, the rate of local cerebral glucose metabolism was also reduced in the contralateral putamen, supporting the notion of cerebral glucose metabolic failure in the lesions (10). The period of cognitive impairment in this patient might coincide with the time of elevated blood glucose episode, although no 18F FDG PET of brain was obtained at that time point. 18F FDG PET was performed at one month after HGHC onset when HGHC did not subside. Considering that PET image was obtained at later clinical stage, decreased cerebral glucose metabolism might be caused by irreversible damage. If PET was performed in comparatively earlier clinical stage, rates of cerebral glucose metabolism might have been higher due to the increased blood flow and high blood glucose.
Our ASL CBF map showed no significant changes. This might suggest the potential transition period from phase of initial autoregulation failure to that of blood vessel damage. Some SPECT studies have suggested that high perfusion of the basal ganglia in the earlier clinical course can be explained by increased CBF due to vascular autoregulation and that low perfusion of the basal ganglia at later clinical course may be caused by neuronal metabolic derangement (1). High blood flow and glucose metabolism in the initial study had changed to low in follow-up study, suggesting that poorly controlled diabetes can cause damage to the blood vessel for a long time.
There are no known relationships of HGHC with malignant lymphoma or capillary telangiectasia within the brain which was regarded as an incidental finding. However, our results should be interpreted with caution. Among multiple brain regions, only six areas of deep gray matters were selected for quantitative analysis. Because this patient's symptom was mainly extrapyramidal in nature, deep gray matters fine-tuning the corticospinal tract motor tone were considered to be significantly involved structures in this HGHC patient. Values of other brain areas might show changes in relation to HGHC. However, a case report study of one patient in compared with healthy controls does not have sufficient statistical power which was regarded as to be beyond the scope of this case report. Therefore, quantitative analysis of only deep gray matter changes related to this extrapyramidal symptom was focused in this study.
In summary, we report an elderly female patient with HGHC. Multi-parametric MRI demonstrated feasibility in measuring myelin loss of brain structures related to HGHC. It might be more useful, especially in a case when visual diagnosis is equivocal.
Figures and Tables
Fig. 1
A 75-year-old female presented with hyperglycemia-induced hemichorea. (a) Body 18F-fludeoxyglucose positron emission tomography (FDG PET) at one year prior demonstrated multifocal nodal masses distributed in the neck, chest, and abdomino-pelvic area, suggesting large B cell lymphoma. She has achieved complete resolution by six sessions of chemotherapy. (b) Non-enhanced CT at time of hyperglycemia (one month before hemichorea) did not show significant abnormality. Conventional MRI at three weeks after hemichorea included T1-weighted image (T1WI) and gadolinium-enhanced T1WI. (c) T1WI of the basal ganglia level demonstrated an area of very subtle hyperintensity involving the right head of putamen with no surrounding edema. (d) Gadolinium-enhanced T1WI revealing enhancement in the right putamen.
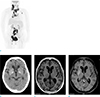
Fig. 2
Time table of symptoms and imaging studies. At a month before hemichorea occurrence, non-ketotic hyperglycemia developed. It was controlled by insulin within 10 days. Non-contrast brain CT was obtained at that time. At 10 days after hemichorea occurrence, first MRI was performed including multi-parametric image acquisition. At three weeks, second MRI including gadolinium-enhanced T1WI enabled suspicion of non-ketotic hyperglycemia-induced hemichorea syndrome in correlation with clinical findings. Third MRI including multi-parametric image was performed at one month after PET acquisition. Multi-parametric quantitative MRIs (first and third) were retrospectively reconstructed and analyzed. Hemichorea persisted after PET acquisition at one month after symptom onset.
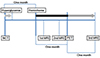
Fig. 3
Quantitative T1, T2, and proton density (PD) maps synthesized from multi-parametric MRI in correlation with arterial spin labeling cerebral blood flow (ASL CBF) map and FDG PET. (a) T1 map of the brain showing very subtle low signal intensity in the right putamen as compared to the left side. (b) T2 map and (c) PD map showing no signal difference between right and left brain structures. (d) ASL CBF revealing no cerebral blood flow abnormality. (e) On FDG PET, local cerebral glucose metabolism was decreased in the contralateral putamen to chorea, supporting the notion of cerebral glucose metabolic failure in lesions.
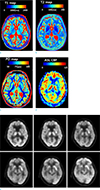
Fig. 4
Multi-parametric quantitative MRI measuring myelin loss in hyperglycemia-induced hemichorea. (a) Myelin-water-fraction (MWF) map was reconstructed from multi-parametric MRI with pulse sequence with quantification of relaxation times and proton density (PD) by multi-echo acquisition of a saturation-recovery using turbo spin-echo readout. (b) Synthetic T1, T2, PD, R1, R2, and MWF map of hyperglycemia-induced hemichorea showing no major difference between right and left brain structures. To obtain average values of normal controls, six age- and sex-matched normal volunteers without any neurologic deficit were registered into Montreal Neurological Institute template and values of predetermined area in putamen (put), pallidum (pall), caudate nucleus (caud), thalamus (thal), red nucleus (RN), and substantia nigra (SN) were averaged. MWFs were markedly reduced in the caudate nucleus, thalamus, substantia nigra, and red nucleus compared to average values of normal controls. On follow-up imaging, loss of MWF was slightly restored in substantia nigra and red nucleus in accordance with mild symptomatic improvement. On the other hand, it seemed that MWFs were further decreased in thalamus. avg-hc = averaged healthy control group; fu = follow up
Acknowledgments
This work was supported by a grant (NRF-2017R1D1A1B03030700) of the National Research Foundation of Korea (NRF) funded by the Korean Government.
References
1. Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002; 200:57–62.
2. Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004; 25:975–976.
3. Nath J, Jambhekar K, Rao C, Armitano E. Radiological and pathological changes in hemiballism-hemichorea with striatal hyperintensity. J Magn Reson Imaging. 2006; 23:564–568.

4. Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol. 2002; 59:448–452.
5. Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med. 2008; 60:320–329.

6. Hagiwara A, Warntjes M, Hori M, et al. SyMRI of the brain: rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic brain segmentation, and myelin measurement. Invest Radiol. 2017; 52:647–657.
7. Hagiwara A, Hori M, Yokoyama K, et al. Utility of a Multiparametric quantitative MRI model that assesses myelin and edema for evaluating plaques, periplaque white matter, and normal-appearing white matter in patients with multiple sclerosis: a feasibility study. AJNR Am J Neuroradiol. 2017; 38:237–242.

8. Warntjes M, Engstrom M, Tisell A, Lundberg P. Modeling the presence of myelin and edema in the brain based on multi-parametric quantitative MRI. Front Neurol. 2016; 7:16.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download