Abstract
Piriformis syndrome caused by an accessory belly of the piriformis muscle is very rare. Only a few cases have been reported. Here, we report a case of piriformis syndrome resulting from an extremely rare type of accessory belly of the piriformis muscle originated at the proximal third portion of the main piriformis muscle and attached separately to the greater trochanter inferior to the insertion of the main piriformis muscle. A definitive diagnosis of piriformis syndrome was made based on magnetic resonance imaging and magnetic resonance neurography findings that were consistent with results of nerve conduction study and needle electromyography.
Piriformis syndrome is a rare entrapment neuropathy in which the sciatic nerve is compromised by an abnormal piriformis muscle (1). It is usually caused by an abnormal condition of the piriformis muscle such as hypertrophy, spasm, contracture, inflammation, or anatomic variation (12). An accessory piriformis muscle is one such anatomic variation. Although possible anatomical relationships between sciatic nerve and piriformis muscle were classified by Beaton and Anson in 1937 into six types (3), only a few cases have been reported regarding the accessory piriformis muscle. Most of previous studies describing the accessory piriformis muscle (1456) reported one of the six types described by Beaton and Anson (3). Ravindranath et al. (7) and Natsis et al. (8) have reported a few cases not classified by Beaton and Anson (3). Here, we report a very rare case of an accessory piriformis muscle not described in the Beaton and Anson classification as a cause of piriformis syndrome. Its magnetic resonance imaging (MRI) and magnetic resonance neurography (MRN) imaging findings are also described.
A 24-year-old female was referred to our institution complaining of a nineteenth-month history of allodynia at the lateral side of her calf and the dorsum of her foot on the left side. In order to exclude disc abnormalities, a lumbar spine MRI was initially performed at an outside local hospital. A mild degenerative bulging disc at L4–5 and L5-S1 levels was noted on sagittal T2-weighted image of the lumbar spine MRI (Fig. 1). Nerve conduction study (NCS) and needle electromyography (EMG) test were performed to confirm the presence of peripheral nerve injury. Sensory NCS revealed no sensory nerve action potential response in left superficial peroneal, left deep peroneal, or left sural nerves. In motor NCS, the left tibial nerve showed low compound motor action potentials while the left peroneal nerve showed no response. EMG revealed abnormal spontaneous activities in biceps femoris, tibialis anterior, and peroneus longus muscles. Electrophysiologic assessment demonstrated abnormalities in muscles innervated by the left sciatic nerve. She was diagnosed with left sciatic neuropathy. No abnormalities were found on EMG performed at different levels (L4, L5) of paraspinal muscles. The patient underwent pelvic bone MRI with MRN for further evaluation. Coronal T1-weighted MR images showed hypertrophy of the left piriformis muscle with an accessory muscle belly lying at the inferior and originating at the proximal third portion of the main piriformis muscle (Fig. 2a). The accessory piriformis muscle was directed downward and laterally. It inserted separately into the greater trochanter, inferior to the insertion of main piriformis muscle on axial T1-weighted MR image (Fig. 2b). Increased size and increased signal intensity of the infrapiriformis portion of the left sciatic nerve were noted on sagittal T2-weighted (Fig. 2c) and axial fat-suppressed T2-weighted MR images (Fig. 2d). On MRN, oblique coronal three-dimensional maximum intensity projection image obtained from fat-suppressed T2-weighted fast field echo showed abnormal thickening of the infra-piriformis portion of left sciatic nerve with concomitant hypertrophy of the left piriformis muscle (Fig. 2e). Coronal diffusion-weighted image with b value of 800 s/mm2 showed thickening of the left sciatic nerve with restricted diffusion (Fig. 2f). Axial T1-weighted (Fig. 2g) and axial fat-suppressed T2-weighted MR images (Fig. 2h) showed no abnormal signal intensity, suggesting denervation of muscles innervated by the left sciatic nerve. MRI and MRN imaging findings were consistent with piriformis syndrome. After the diagnosis, she was treated conservatively with analgesics. Her symptom slightly improved. Surgical treatment was considered for refractory case. However, the patient was lost to follow-up. Thus, no further evaluation was done.
Although piriformis muscle as an etiologic factor for sciatic pain and lower back pain was first described by Yeoman (9) in 1928, correct diagnosis of piriformis syndrome is often delayed and frequently misdiagnosed due to its rarity. In 1937, Beaton and Anson (3) classified possible anatomical relationships between sciatic nerve and piriformis muscle into six types (Fig. 3a–f). However, only a few cases of anatomic variation of the piriformis muscle have been reported as a cause of piriformis syndrome, especially regarding the accessory piriformis muscle. Lee et al. (1) and Sen and Rajesh (4) have reported accessory muscle fibers of piriformis muscles extending medially over the sacral foramen and crossing over the sacral nerves. These corresponded to a type D variation (Fig. 3d). Yadav et al. (5) and Polesello et al. (6) have reported two distinct bellies of piriformis muscles that are fused to form a common tendon and inserted to the greater trochanter. As a nerve branch of the sciatic nerve passed between the accessory belly and the main piriformis muscle in each case, these cases were type B variations (Fig. 3b). However, there are also a few case reports of the accessory piriformis muscle not described in the Beaton and Anson classification. Ravindranath et al. (7) have reported three cadaver cases of accessory piriformis muscle originated from the sacrotuberous ligament or the fascia overlying the gluteus medius. Accessory slips of these cases merged with the main tendinous part of piriformis muscles. However, the sciatic nerve was deeply related to the accessory slip and the main piriformis muscle. Natsis et al. (8) have reported an extremely rare cadaver case of anatomical variation, showing that the piriformis muscle has three muscle bellies. Unlike other cases described previously, our patient had an accessory belly of the piriformis muscle which originated at the inferior aspect and the proximal third portion of the main piriformis muscle, coursed deep to the sciatic nerve, and attached separately into the greater trochanter (Fig. 3g). In addition, coexisting hypertrophy of the piriformis muscle was noted as an additional cause of piriformis syndrome. This is an extremely rare anatomic variation. To the best of our knowledge, such case has not been previously reported.
Although the diagnosis of piriformis syndrome is challenging due to its nonspecific clinical symptoms and the absence of definitive diagnostic tests, MRI can be used to make a confirmative diagnosis of suspected piriformis syndrome by detecting abnormal anatomy of the affected nerve and evaluating the size and anatomic variation of the piriformis muscle (12). In our patient, an accessory belly and hypertrophy of the left piriformis muscle were noted on the patient's MRI. In addition, increased size and increased signal intensity of the infra-piriformis portion of left sciatic nerve were seen on T2-weighted MRI images and MRN. These were sufficient for an imaging diagnosis of piriformis syndrome, consistent with results of the NCS and EMG. However, the present case has not yet been surgically-confirmed as the initial treatment for piriformis syndrome is conservative. Surgical release of the sciatic nerve and sectioning of the accessory belly of the piriformis muscle may be considered in refractory cases (2).
In conclusion, an accessory belly of the piriformis muscle is a rare but important etiology of piriformis syndrome. Radiologists in particular must be aware of this etiology because early recognition using MRI may prevent delayed diagnosis of piriformis syndrome.
Figures and Tables
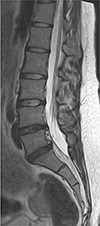 | Fig. 1Sagittal T2-weighted MR image (repetition time [TR] = 2750 ms, echo time [TE] = 104 ms) of the lumbar spine showing a mild degenerative bulging disc at L4-5 and L5-S1 levels. |
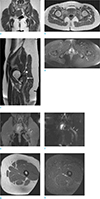 | Fig. 2(a) Coronal T1-weighted MR image (TR = 691 ms, TE = 14 ms) revealing hypertrophy (asterisk) of the left piriformis muscle with an accessory belly (arrow) appearing at the inferior, proximal-third portion of the main piriformis muscle which coursed downward and laterally. (b) Axial T1-weighted MR image (TR = 709 ms, TE = 19 ms) showing the accessory belly (arrow) of the left piriformis muscle attaching to the greater trochanter, inferior to the insertion of the main piriformis muscle. (c) Sagittal T2-weighted MR image (TR = 3992 ms, TE = 100 ms) showing the accessory belly (arrowhead) at the inferior aspect of the left piriformis muscle along with thickening of the infrapiriformis portion of the left sciatic nerve (arrow). (d) Axial fat-suppressed T2-weighted MR image (TR = 4385 ms, TE = 70 ms) showing increased size and increased signal intensity of the left sciatic nerve (arrow). (e) Oblique coronal three-dimensional maximum intensity projection image obtained from fat-suppressed T2-weighted fast field echo (TR = 8 ms, TE = 4 ms, flap angle = 30°) clearly showing an abnormally thick left sciatic nerve (arrows) inferior to the hypertrophic left piriformis muscle (asterisk). (f) Coronal diffusion-weighted image with b value of 800 s/mm2 clearly demonstrating an abnormally thick left sciatic nerve (arrows) with diffusion restriction. (g, h) Axial T1-weighted (TR = 709 ms, TE = 19 ms) and axial fat-suppressed T2-weighted MR images (TR = 4385 ms, TE = 70 ms) showing no abnormal signal intensity suggesting denervation of left biceps femoris, semitendinosus, semimembranosus, and adductor magnus that are innervated by the left sciatic nerve. |
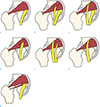 | Fig. 3Drawings illustrating the six types of the Beaton and Anson's classification and our case regarding anatomical variation between the piriformis muscle and he sciatic nerve. Colored red and yellow indicate piriformis muscles and sciatic nerves (and its divisions), respectively. (a) Type A, undivided sciatic nerve exits the pelvis below the piriformis muscle. (b) Type B, sciatic nerve divides in the pelvis, common peroneal nerve pierces the piriformis muscle, and tibial nerve courses deep to the piriformis muscle. (c) Type C, sciatic nerve divides in the pelvis, common peroneal nerve courses over the piriformis muscle, and tibial nerve courses deep to the piriformis muscle. (d) Type D, undivided sciatic nerve exits the pelvis and pierces the piriformis muscle. (e) Type E, sciatic nerve divides in the pelvis, common peroneal nerve courses over the piriformis muscle, and tibial nerve pierces the piriformis muscle. (f) Type F, undivided sciatic nerve exits the pelvis and courses over the piriformis muscle. (g) Our case, accessory belly appears at the inferior aspect and proximal third portion of the main piriformis muscle, coursing laterally and inserting into the greater trochanter separately. Undivided sciatic nerve exits the pelvis below the main piriformis muscle and over the accessory belly. |
References
1. Lee EY, Margherita AJ, Gierada DS, Narra VR. MRI of piriformis syndrome. AJR Am J Roentgenol. 2004; 183:63–64.

2. Petchprapa CN, Rosenberg ZS, Sconfienza LM, Cavalcanti CF, Vieira RL, Zember JS. MR imaging of entrapment neuropathies of the lower extremity. Part 1. The pelvis and hip. Radiographics. 2010; 30:983–100.
3. Beaton LE, Anson BJ. The relation of the sciatic nerve and its subdivisions to the piriformis muscle. Anat Rec. 1937; 70:1–5.
4. Sen A, Rajesh S. Accessory piriformis muscle: an easily identifiable cause of piriformis syndrome on magnetic resonance imaging. Neurol India. 2011; 59:769–771.

5. Yadav Y, Mehta V, Roy S, Suri R, Rath G. Superior gluteal nerve entrapment between two bellies of piriformis muscle. IJAV. 2010; 3:203–204.
6. Polesello GC, Queiroz MC, Linhares JPT, Amaral DT, Ono NK. Anatomical variation of piriformis muscle as a cause of deep gluteal pain: diagnosis using MR neurography and treatment. Rev Bras Ortop. 2013; 48:114–117.

7. Ravindranath Y, Manjunath KY, Ravindranath R. Accessory origin of the piriformis muscle. Singapore Med J. 2008; 49:e217–e218.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download