Abstract
Undifferentiated pleomorphic sarcoma (UPS) arising from the descending thoracic aorta is a rare type of tumor. To our knowledge, only a few cases have been reported in the literature. We present computed tomography (CT) and magnetic resonance imaging findings of a 43-year-old male patient with undifferentiated pleomorphic sarcoma of the descending thoracic aorta, which showed enhancement on only magnetic resonance imaging (MRI). MRI with contrast enhancement may be useful in differentiating an aortic tumor from atherosclerotic disease.
Primary sarcomas of large systemic arteries are uncommon, representing less than 1% of all sarcomas (12). The clinical presentation is usually nonspecific and mostly related to tumor embolism resulting in limb or visceral ischemia (1). Due to their low prevalence and nonspecific presentations, the diagnosis is often only made postmortem or late in the course of the disease in most cases. Among these entities, undifferentiated pleomorphic sarcoma (UPS), previously called malignant fibrous histiocytoma (MFH), has been defined as a group of pleomorphic, high-grade sarcoma that shows no definable line of differentiation when using ancillary techniques (34).
Herein, we present a rare case of UPS arising from the descending thoracic aorta with emphasis on magnetic resonance imaging (MRI) findings, initially diagnosed as a pseudoaneurysm with periaortic hematoma on computed tomography (CT).
A 43-year-old male patient visited the outpatient clinic of cardiothoracic surgery department with chest pain and epigastric discomfort for several months. The patient had been previously healthy. The results of all laboratory tests including blood chemistry, coagulation studies, and cardiac enzymes were within normal ranges. At that time, an electrocardiogram showed normal sinus rhythm.
Pre- and post-contrast enhanced chest CT were performed for initial image workup, using 128-row detector CT scanner (Somatom Definition AS, Siemens, Erlangen, Germany). CT showed a heterogeneous attenuated paraaortic mass encasing the right aspect of the descending thoracic aorta at level of T7 to T9, and measuring 6.0 × 4.2 × 5.4 cm in size (Fig. 1a, b). The mass was firmly attached to the descending thoracic aorta with suspicious minimal luminal narrowing, and subtle irregularity of the aortic lumen. Also, there was a small ulcer-like projection at the inferior portion of the mass (Fig. 1c). On contrast-enhanced CT, there was no significant enhancement. The radiologic appearance resembled a pseudoaneurysm of the aorta with periaortic hematoma formation. But hypovascular tumor could not be excluded, because there was no evidence of trauma history or atherosclerotic change of the remaining part of the aorta.
Thoracic MRI on a 3T system (Skyra, Siemens, Erlangen, Germany) was performed for further evaluation. All examinations included T1-weighted, T2-weighted and preand post-dynamic contrast-enhanced fat suppressed T1 weighted sequences (immediate, 3-minute and 5-minute). After the examination, the subtraction images were made automatically on a pixel-by-pixel basis.
The mass showed iso signal intensity (SI) on T1-weighted image with areas of high SI representing hemorrhagic components (Fig. 2a). On T2-weighted image, the mass showed heterogeneous SI (Fig. 2b). On contrast-enhanced MRI after intravenous injection of 15 mL (0.1 mmol/kg) of gadoterate meglumine (Dotarem, Guerbet, Cedex, France), the mass showed peripheral rim and areas of small nodular enhancement, which were not shown on CT (Fig. 2c–f). The loss of fat plane between the mass and aorta with irregularity of aortic wall was better depicted on MRI than on CT. Also, the existence of fat plane between the mass and surrounding structures was better demonstrated on MRI with no invasion into adjacent vertebral bodies or ribs. Based on these MRI findings, the mass was considered to be a tumor.
For pathologic confirmation, video-assisted thoracoscopic surgical (VATS) lung biopsy was performed. The result showed a high-grade sarcoma of undetermined type. For evaluation of distant metastasis, positron emission tomography-computed tomography (PET-CT) was subsequently performed. The mass showed FDG (fluorodeoxyglucose) hypermetabolism on the periphery of the mass (SUVmax, 10.32), corresponding to enhancing portions on MRI (Fig. 3a). And there was no evidence of distant metastasis on PET-CT.
At thoracotomy, about 6 cm-sized mass invading the descending thoracic aorta was noted. Concomitant tumor excision and replacement of the thoracic aorta using an artificial vascular graft (Hemashield) were performed.
On a review of the histopathologic examination, the tumor cells were observed in the aortic wall with extraluminal perivascular invasion. The tumor was composed of short spindle pleomorphic cells with prominent nucleoli and high mitotic rates (Fig. 3b). The immunohistochemical stain showed positive results in vimentin, and negative results in S100, CD34, desmin, smooth muscle actin (SMA) staining. Considering these morphologic and immunohistochemical stain results, the tumor was compatible with UPS of the descending thoracic aorta.
Because resection margin was positive, subsequent chemotherapy (Doxorubicin and olaratumab) was selected for treatment. However, MRI obtained 2 months after surgery showed that regional tumor recurrence with bone destruction at T7 vertebral body and distant metastasis in both lungs and left pleura. The patient has been treated with palliative radiation therapy after surgery.
Primary aortic sarcoma is a rare disease entity and most commonly occurs in the descending thoracic aorta (35%), followed by abdominal aorta (27%), thoracoabdominal aorta (27%), and ascending aorta or aortic arch (11%), in descending order (5). Metastasis occurs most frequently in the lung, bone and adrenal gland.
In 1985, Wright et al. (6) proposed a clinicopathologic classification, categorizing of primary aortic sarcomas as intimal and mural types. And Thalheimer et al. (7) further subclassified intimal sarcomas into intimal angiosarcomas of endothelial origin and myofibroblastic sarcomas of mesenchymal origin based on the immunohistochemical findings. Intimal type often forms polypoid masses in the aortic lumen, causing obstruction of aorta and its branches. As a result, ischemia of the organs with or without mechanical obstruction is likely to occur. Mural type originates in the media or adventitia and usually invades the surrounding structure with limited metastasis (8). This type manifests as an extraluminal growth pattern and extends to surrounding tissue, mimicking atherosclerotic or inflammatory diseases (24). In the present case, the tumor involved all three layers, which showed extramural growth pattern, and no evidence of filling defect within the lumen. Highly invasive and poorly differentiated sarcomas in advanced stage cannot be included in this classification, because they are detected with invasion of all three layers (5).
Aortic tumors can also be classified by their histologic subtype (e.g. sarcoma, MFH, angiosarcomas, leiomyosarcomas, and fibrosarcomas) (8). There is no specific imaging finding to differentiate the various type of aortic tumors. Generally, CT shows irregular large soft tissue density mass originating from the aorta with heterogeneous enhancement and rapid growth (389). MRI usually shows a mass with nonspecific SI characteristics on T1-and T2-weighted images (8). With contrast-enhancement, MRI is superior to CT in differentiating an aortic tumor from atherosclerotic disease, due to its high contrast resolution. Additionally, MRI has an advantage in illustrating the relationship between the tumor and its neighboring structures, and may allow for better preoperative planning (28). There is no report on PET-CT findings of UPS occurring in the aorta, but von Flack et al. (2) reported that primary malignant sarcomas of the great vessels show FDG hypermetabolism on PET-CT. Similarly, our case showed FDG hypermetabolism at the peripheral solid portions of the mass.
Because primary aortic tumors are rare, they are infrequently suspected and difficult to diagnose in clinical practice. Radiologic evaluation plays an important role in diagnosis, but it can be difficult to differentiate an aortic tumor from atherosclerotic disease based on their radiological appearances. Similarly, in our case, it was difficult to distinguish them initially because there was no significant enhancement on CT imaging. MRI is particularly beneficial in evaluating for tumorous condition in the case of indeterminate lesions. Because avid peripheral rim and small nodular enhancement were seen on MR images, a diagnosis of aortic sarcoma could confidently be made in this case. The ulcer-like projection was actually tumor erosion, not an atherosclerotic ulcer. In our case, MRI was useful in differentiating an aortic sarcoma from other differential diagnoses. Also, PET-CT was helpful for obtaining a diagnosis because of hypermetabolic activity of the lesion.
The standard treatment protocol of UPS of the aorta has not been clearly established. Rusthoven et al. (10) reported that the current gold standard treatment for UPS of the aorta is en bloc surgical resection of the tumor-involved aorta and graft interposition. Adjuvant chemotherapy and radiotherapy can be used for embolic, metastatic or nonresectable disease (8).
In summary, we report a rare case of a 43-year-old man with UPS arising from the descending thoracic aorta. Because of its rarity, poor prognosis and very low survival rate, an early diagnosis is essential. However, it can be often difficult to differentiate hypovascular aortic tumor from atherosclerotic disease, especially when the lesion shows no enhancement on CT. In such cases contrast-enhanced MRI can be helpful in distinguishing them due to its higher contrast resolution, as in our case.
Figures and Tables
Fig. 1
Chest CT images of a 43-year-old man with undifferentiated pleomorphic sarcoma of the descending thoracic aorta. Axial pre- (a) and post-contrast enhanced (b, c) images show heterogeneous attenuated mass broadly attached to the descending thoracic aorta (white arrows). The mass shows no significant enhancement on CT images. There is a small ulcer-like projection at the inferior portion of the mass (black arrow on c).
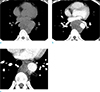
Fig. 2
Thoracic MR images of the patient. Axial T1-weighted (a) and T2-weighted (b) images show the mass of the descending thoracic aorta with heterogeneous signal intensity (white arrows). Axial T1-weighted, fat-saturated pre- (c) and post-contrast image (d), subtraction images (e, f) show peripheral rim and small nodular enhancement of the mass (white arrows), which was not shown on CT images.
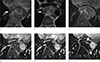
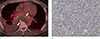
Fig. 3
PET-CT and microscopic findings of the patient. Axial PET-CT image (a) shows the FDG hypermetabolism on the periphery of the mass (white arrows), corresponding to enhancing portions on MRI. The pathologic finding (b) shows that the tumor is composed of short spindle pleomorphic cells with prominent nucleoli and high mitotic rates (Hematoxylin & Eosin stain, × 40).
References
1. Sebenik M, Ricci A Jr, DiPasquale B, et al. Undifferentiated intimal sarcoma of large systemic blood vessels: report of 14 cases with immunohistochemical profile and review of the literature. Am J Surg Pathol. 2005; 29:1184–1193.
2. von Falck C, Meyer B, Fegbeutel C, et al. Imaging features of primary sarcomas of the great vessels in CT, MRI and PET/CT: a single-center experience. BMC Med Imaging. 2013; 13:25.

3. Heo SY, Park CS, Kim SJ, Park NH, Heo JH, Lee JJ. Undifferentiated pleomorphic sarcoma of the thoracic aorta: a case report. J Korean Soc Radiol. 2016; 75:304–308.

4. Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol. 2014; 27:Suppl 1. S39–S46.

5. Chiche L, Mongredien B, Brocheriou I, Kieffer E. Primary tumors of the thoracoabdominal aorta: surgical treatment of 5 patients and review of the literature. Ann Vasc Surg. 2003; 17:354–364.

6. Wright EP, Glick AD, Virmani R, Page DL. Aortic intimal sarcoma with embolic metastases. Am J Surg Pathol. 1985; 9:890–897.

7. Thalheimer A, Fein M, Geissinger E, Franke S. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J Vasc Surg. 2004; 40:548–553.

8. Utsunomiya D, Ikeda O, Ideta I, Hirayama T, Yamashita Y, Kamio T. Malignant fibrous histiocytoma arising from the aortic wall mimicking a pseudoaneurysm with ulceration. Circ J. 2007; 71:1659–1661.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download