Abstract
Purpose
The purpose of this study was to identify the effect of ghrelin on memory impairment in a rat model of vascular dementia induced by chronic cerebral hypoperfusion.
Methods
Randomized controlled groups and the posttest design were used. We established the representative animal model of vascular dementia caused by bilateral common carotid artery occlusion and administered 80 μg/kg ghrelin intraperitoneally for 4 weeks. First, behavioral studies were performed to evaluate spatial memory. Second, we used molecular biology techniques to determine whether ghrelin ameliorates the damage to the structure and function of the white matter and hippocampus, which are crucial to learning and memory.
Results
Ghrelin improved the spatial memory impairment in the Y-maze and Morris water maze test. In the white matter, demyelination and atrophy of the corpus callosum were significantly decreased in the ghrelin-treated group. In the hippocampus, ghrelin increased the length of hippocampal microvessels and reduced the microvessels pathology. Further, we confirmed angiogenesis enhancement through the fact that ghrelin treatment increased vascular endothelial growth factor (VEGF)-related protein levels, which are the most powerful mediators of angiogenesis in the hippocampus.
Conclusion
We found that ghrelin affected the damaged myelin sheaths and microvessels by increasing angiogenesis, which then led to neuroprotection and improved memory function. We suggest that further studies continue to accumulate evidence of the effect of ghrelin. Further, we believe that the development of therapeutic interventions that increase ghrelin may contribute to memory improvement in patients with vascular dementia.
Go to : 
References
1. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013; 80(4):844–866. https://doi.org/10.1016/j.neuron.2013.10.008.

2. Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018; 134(B):226–239. https://doi.org/10.1016/j.neuropharm.2017.12.030.

3. Ma X, Sun Z, Liu Y, Jia Y, Zhang B, Zhang J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regeneration Research. 2013; 8(22):2050–2059. https://doi.org/10.3969/j.issn.1673-5374.2013.22.004.
4. Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, et al. Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke. 2011; 42(5):1345–1350. https://doi.org/10.1161/strokeaha.110.600825.

5. Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011; 42(2):445–452. https://doi.org/10.1161/strokeaha.110.596486.

6. Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, et al. White matter lesions in an unselected cohort of the elderly: Astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathology and Applied Neurobiolo- gy. 2007; 33(4):410–419. https://doi.org/10.1111/j.1365-2990.2007.00828.x.

7. Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neuroscience Letters. 2010; 482(3):235–239. https://doi.org/10.1016/j.neulet.2010.07.046.

8. O’Brien JT, Thomas A. Vascular dementia. The Lancet. 2015; 386(10004):1698–1706. https://doi.org/10.1016/S0140-6736(15)00463-8.

9. Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. Journal of Neurochemistry. 2010; 115(4):814–828. https://doi.org/10.1111/j.1471-4159.2010.06958.x.

10. Baskys A, Hou AC. Vascular dementia: Pharmacological treatment approaches and perspectives. Clinical Interventions in Aging. 2007; 2(3):327–335.
11. Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: A systematic review of efficacy. Dementia and Geriatric Cognitive Disorders. 2010; 30(2):161–178. https://doi.org/10.1159/000316119.

12. Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Trends in Neurosciences. 2011; 34(1):31–40. https://doi.org/10.1016/j.tins.2010.10.001.

13. Park S. Ghrelin. Endocrinology and Metabolism. 2010; 25(4):258–263. https://doi.org/10.3803/enm.2010.25.4.258.

14. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kanga-wa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402(6762):656–660. https://doi.org/10.1038/45230.

15. Moon M, Kim S, Hwang L, Park S. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocrine Journal. 2009; 56(3):525–531. https://doi.org/10.1507/endocrj.K09E-089.

16. Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006; 9(3):381–388. https://doi.org/10.1038/nn1656.

17. Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonel-li TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochemical and Biophysical Research Communications. 2004; 313(3):635–641. https://doi.org/10.1016/j.bbrc.2003.11.150.

18. Liu Y, Chen L, Xu X, Vicaut E, Sercombe R. Both ischemic preconditioning and ghrelin administration protect hippocampus from ischemia/reperfusion and upregulate uncoupling protein-2. BMC Physiology. 2009; 9:17. https://doi.org/10.1186/1472-6793-9-17.

19. Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochemical and Biophysical Research Communications. 2007; 359(3):795–800. https://doi.org/10.1016/j.bbrc.2007.05.192.

20. Liu Y, Wang PS, Xie D, Liu K, Chen L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/ reperfusion. Chinese Journal of Physiology. 2006; 49(5):244–250.
21. Wang P, Xie ZH, Guo YJ, Zhao CP, Jiang H, Song Y, et al. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochemical and Biophysical Research Communications. 2011; 411(3):620–626. https://doi.org/10.1016/j.bbrc.2011.07.003.

22. Lopez NE, Krzyzaniak MJ, Blow C, Putnam J, Ortiz-Pomales Y, Hageny AM, et al. Ghrelin prevents disruption of the blood–brain barrier after traumatic brain injury. Journal of Neurotrauma. 2012; 29(2):385–393. https://doi.org/10.1089/neu.2011.2053.

23. Mead R. The design of experiments: Statistical principles for practical applications. Cambridge: Cambridge University Press;1990. p. 7–8.
24. Spence KW, Lippitt R. An experimental test of the sign-gestalt theory of trial and error learning. Journal of Experimental Psychology. 1946; 36(6):491–502. https://doi.org/10.1037/h0062419.

25. Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982; 297(5868):681–683. https://doi.org/10.1038/297681a0.

26. Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Experimental Neurology. 2015; 272:97–108. https://doi.org/10.1016/j.expneurol.2015.05.006.

27. Kim Y, Kim S, Kim C, Sato T, Kojima M, Park S. Ghrelin is required for dietary restriction-induced enhancement of hippocampal neurogenesis: Lessons from ghrelin knockout mice. Endocrine Journal. 2015; 62(3):269–275. https://doi.org/10.1507/endocrj.EJ14-0436.

28. Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clinical Endocrinology. 2002; 56(2):203–206. https://doi.org/10.1046/j.0300-0664.2001.01456.x.

29. Harvey J. Leptin regulation of neuronal morphology and hippocampal synaptic function. Frontiers in Synaptic Neuroscience. 2013; 5:3. https://doi.org/10.3389/fnsyn.2013.00003.

30. Irving AJ, Harvey J. Leptin regulation of hippocampal synaptic function in health and disease. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014; 369(1633):20130155. https://doi.org/10.1098/rstb.2013.0155.

31. Li E, Kim Y, Kim S, Park S. Ghrelin-induced hippocampal neurogenesis and enhancement of cognitive function are mediated independently of GH/IGF-1 axis: Lessons from the spontaneous dwarf rats. Endocrine Journal. 2013; 60(9):1065–1075. https://doi.org/10.1507/endocrj.ej13-0045.

32. Chen L, Xing T, Wang M, Miao Y, Tang M, Chen J, et al. Local infusion of ghrelin enhanced hippocampal synaptic plasticity and spatial memory through activation of phosphoinositide 3‐ kinase in the dentate gyrus of adult rats. European Journal of Neuroscience. 2011; 33(2):266–275. https://doi.org/10.1111/j.1460-9568.2010.07491.x.
33. Innocenti GM. General organization of callosal connections in the cerebral cortex. Jones EG, Peters A, editors. Sensory-Motor Areas and Aspects of Cortical Connectivity. Boston (MA): Springer US;1986. p. 291–353.

34. Wu XP, Gao YJ, Yang JL, Xu M, Sun DH. Quantitative measurement to evaluate morphological changes of the corpus callosum in patients with subcortical ischemic vascular dementia. Acta Radiologica. 2015; 56(2):214–218. https://doi.org/10.1177/0284185114520863.

35. Lee JY, Oh TH, Yune TY. Ghrelin inhibits hydrogen peroxide-induced apoptotic cell death of oligodendrocytes via ERK and p38MAPK signaling. Endocrinology. 2011; 152(6):2377–2386. https://doi.org/10.1210/en.2011-0090.

36. Neto CJBF, Paganelli RA, Benetoli A, Lima KCM, Milani H. Permanent, 3-stage, 4-vessel occlusion as a model of chronic and progressive brain hypoperfusion in rats: A neurohisto-logical and behavioral analysis. Behavioural Brain Research. 2005; 160(2):312–322. https://doi.org/10.1016/j.bbr.2004.12.016.

37. Jantaratnotai N, Ryu JK, Schwab C, McGeer PL, McLarnon JG. Comparison of vascular perturbations in an Aβ-injected animal model and in AD brain. International Journal of Alzhei- mer’s Disease. 2011; 2011:918280. https://doi.org/10.4061/2011/918280.

38. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407(6801):249–257. https://doi.org/10.1038/35025220.

39. Plate KH. Mechanisms of angiogenesis in the brain. Journal of Neuropathology and Experimental Neurology. 1999; 58(4):313–320. https://doi.org/10.1097/00005072-199904000-00001.

40. Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: A brief review. Journal of Neuroimmune Pharmacology. 2007; 2(3):284–289. https://doi.org/10.1007/s11481-007-9073-3.

41. Pillai A, Mahadik SP. Differential effects of haloperidol and olanzapine on levels of vascular endothelial growth factor and angiogenesis in rat hippocampus. Schizophrenia Research. 2006; 87(1-3):48–59. https://doi.org/10.1016/j.schres.2006.06.017.

42. Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circulation Research. 2002; 90(12):1243–1250. https://doi.org/10.1161/01.res.0000022200.71892.9f.

43. Wang L, Chen Q, Li G, Ke D. Ghrelin stimulates angiogenesis via GHSR1a-dependent MEK/ERK and PI3K/Akt signal pathways in rat cardiac microvascular endothelial cells. Peptides. 2012; 33(1):92–100. https://doi.org/10.1016/j.peptides.2011.11.001.

Go to : 
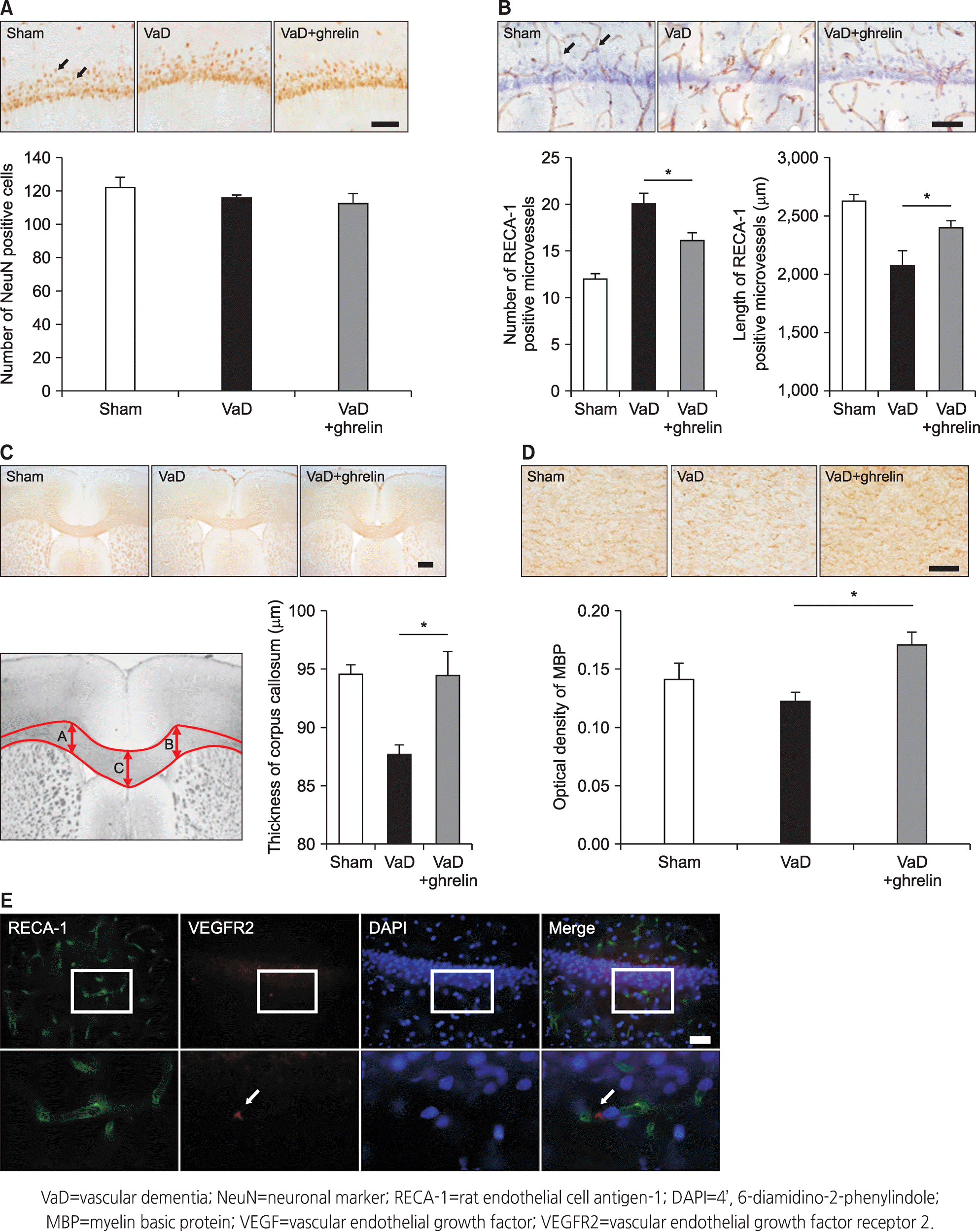 | Figure 2.Results of the immunohistochemistry analysis. (A) Staining by NeuN in the hippocampus. (B) Staining by RECA-1 in the hippocampus. (C) Photomicrographs of callosal thickness in the corpus callosum. (D) Staining by MBP in the corpus callosum. (E) Double immunofluorescence staining for VEGF and RECA-1 demonstrates their localization in the hippocampus. Scale bar represents 100 μm. * means p<.05 when compared to the VaD group. |
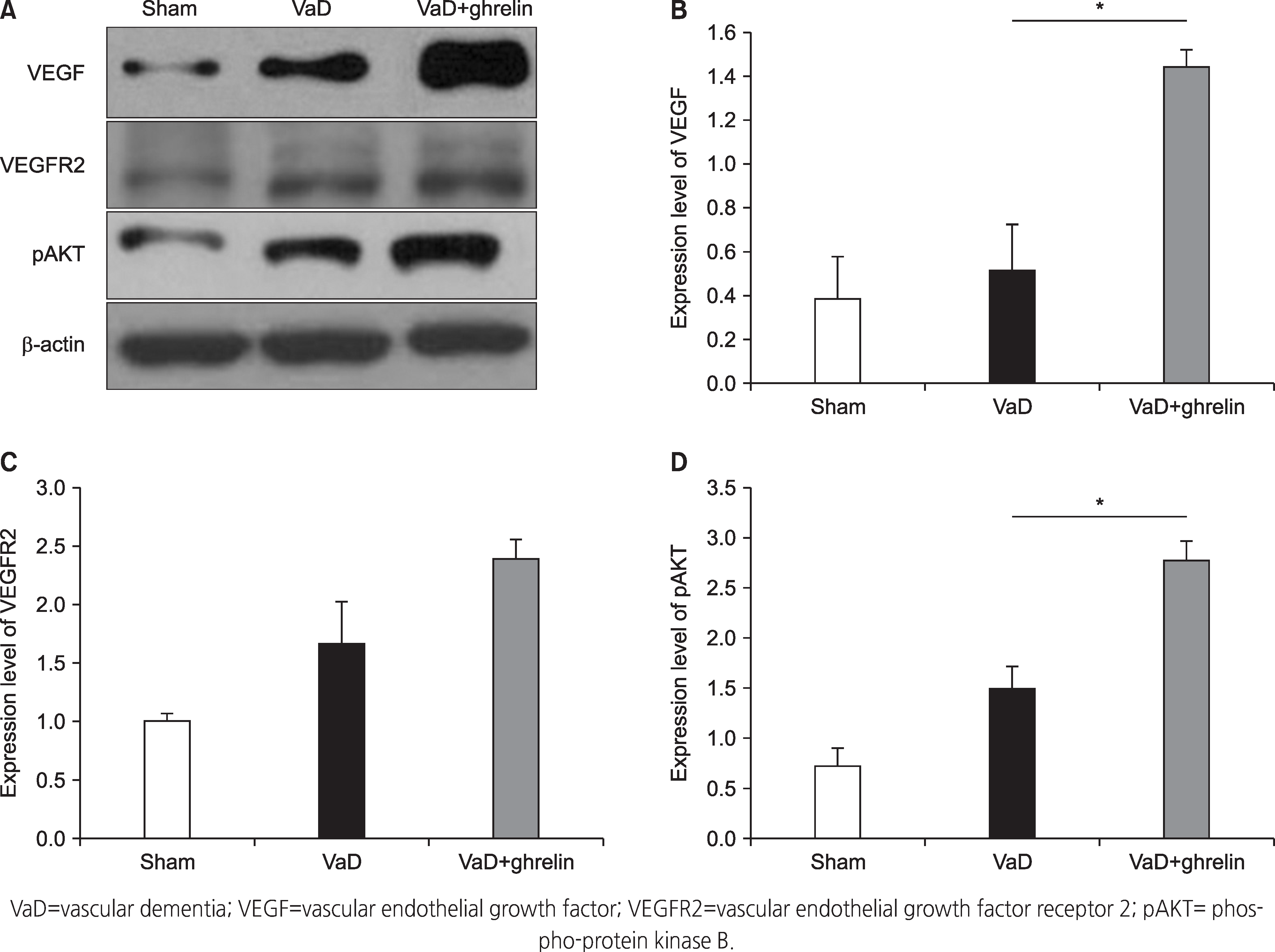 | Figure 3.Result of western blot analysis. (A) Western blot analysis of VEGF, VEGFR2, and pAKT. (B) Expression level of VEGF in the hippocampus. (C) Expression level of VEGFR2 in the hippocampus. (D) Expression level of pAKT in the hippocampus. * means p<.05 when compared to the VaD group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download