Dear Editor,
The fecal occult blood test (FOBT) has been widely used to screen for colorectal cancer. However, because of the low sensitivity and false-positive reactions of traditional guaiac-based tests, fecal immunochemical tests (FITs) that use specific antibodies to detect human Hb are more frequently used in clinical laboratories [1]. External quality assessment (EQA) trials, an essential component of a laboratory's quality management system, verify that laboratory results meet quality expectations required for patient care [2].
In Korea, approximately 70% of participants in EQA trials perform qualitative FITs [3]. In the 2015 EQA trials, only 11% of participants using a qualitative FIT received an “acceptable” result for the negative sample (FOB B2-QL), which was adjusted to 4 µg Hb/g feces. Most qualitative FITs showed positive results when the sample contained >10 µg Hb/g feces, according to the manufacturers' instructions. These are likely to be false-positive results because of the low cutoff concentration of these FITs. Thus, after the 2015 EQA trials, we provided feedback on the unacceptable EQA results to the manufacturers and requested them to verify the cutoff concentration of the qualitative FITs. In the 2016 EQA trials, all qualitative FIT results exhibited accuracy rates >90% [4]. We assessed whether there was an improvement in six qualitative FITs popular in Korea, by checking their cutoff concentration pre- and post-feedback. This study was conducted in accordance with the Declaration of Helsinki (2013 version) and approved by the Institutional Review Board of Keimyung University Dongsan Hospital, Daegu, Korea (DSMC 2018-03-042).
The evaluation of all FITs followed the Faecal immunochemical TesTs for Haemoglobin Evaluation Reporting (FITTER) standard [5]. We used residual fecal samples that had been submitted to two tertiary medical centers (Daegu Catholic University Medical Center, Daegu and Keimyung University Dongsan Hospital, Daegu) in Korea for diagnostic quantitative FITs in March 2016 and March 2018. The quantitative FIT was performed using OC-Sensor DIANA (Eiken Chemical Co., Tokyo, Japan). Sample collection, storage, preparation, and analysis were performed according to the manufacturer's instructions. Samples with an Hb concentration ≤20 µg/g feces were collected to check the cutoff concentrations of qualitative FITs.
Six qualitative FITs were used: Eiken Hemocatch light (Eiken Chemical Co.), ASAN Easy Test FOB (Asan Pharmaceutical, Seoul, Korea), YD OcculTech FOB test (YD Diagnostics, Yongin, Korea), Bio Focus FOB Rapid test (Bio Focus Co., Uiwang, Korea), SD FOB Rapid test (Standard Diagnostics, Seoul, Korea), and Humasis FOB test (Humasis Co., Anyang, Korea). All test processes were performed according to the manufacturers' instructions. The cutoff concentration of six qualitative FITs was 10 µg Hb/g feces in each instruction, but there was no definite information on the target population for the cutoff.
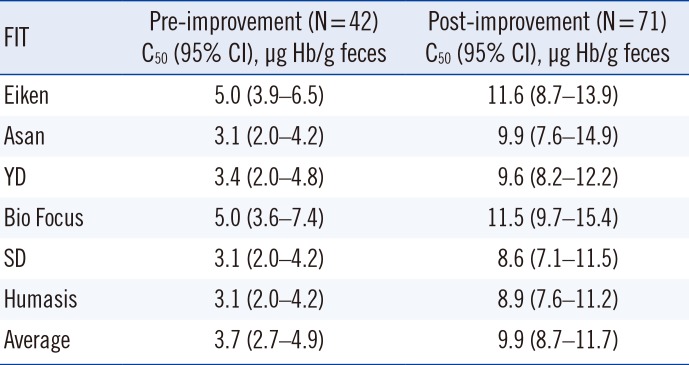
Pre-improvement data were obtained from 42 samples (0.4–17 µg Hb/g feces) tested with qualitative FITs at Daegu Catholic University Medical Center in 2016 [3]. To obtain post-improvement data, 71 samples (0.2–19.6 µg Hb/g feces) were tested with qualitative FITs at Keimyung University Dongsan Hospital. We used probit regression to estimate the concentration corresponding to 50% positive results (C50) close to the cutoff concentration stated by the manufacturers (10 µg Hb/g feces) [6]. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). The average C50 for all pre- and post-improvement qualitative FITs was 3.7 µg Hb/g feces and 9.9 µg Hb/g feces, respectively (Table 1).
Many potential problems can be identified by unacceptable EQA results based on the CLSI guideline for clinical laboratories [7]. To rule out potential problems with testing reagents, we checked the qualitative FIT performance pre- and post-improvement based on analyses of patient fecal samples and found that the unacceptable results were due to reagent problems.
Qualitative FITs are specific to human Hb and easy to use in clinical laboratories. The United States Food and Drug Administration and the European Commission developed strict standards and guidelines for FOBTs in 2007 and 2010, respectively [89]. However, the Korean Ministry of Food and Drug Safety established guidelines for performance testing methods for FOBT reagents with reference to some CLSI guidelines (EP07-A2, EP12-A2 and EP17-A2) only in 2018; these detail how to validate the cutoff concentration [10]. All FOBT reagents should be registered with the Korean Ministry of Food and Drug Safety once the assessment specified in these guidelines has been conducted.
In summary, we discovered the problem with the qualitative FIT reagents used via EQA trials prior to rigorous regulation by the relevant authorities and confirmed that the qualitative FITs improved after feedback. EQA participation will help identify problems in laboratory practices, allowing for appropriate corrective action at the individual laboratory level. Furthermore, EQA trials are useful for recognizing and solving fundamental problems.
References
1. Inadomi JM. Screening for colorectal neoplasia. N Engl J Med. 2017; 376:149–156. PMID: 28076720.
2. Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 2011; 57:1670–1680. PMID: 21965556.
3. Jeon CH, Lee AJ, Kim KD. The Urinalysis and Routine Microscopy Subcommitte, Korean Association of External Quality Assessment Service. Annual report on the external quality assessment scheme for urinalysis and faecal occult blood testing in Korea (2015). J Lab Med Qual Assur. 2016; 38:120–128.
4. Jeon CH, Lee AJ, Kim SG, Suh HS, Bae YC. Annual report on the external quality assessment scheme for urinalysis and faecal occult blood testing in Korea (2016). J Lab Med Qual Assur. 2017; 39:117–123.
5. Fraser CG, Allison JE, Young GP, Halloran SP, Seaman H. A standard for faecal immunochemical tests for haemoglobin evaluation reporting (FITTER). Ann Clin Biochem. 2014; 51:301–302. PMID: 24345727.
6. Åsberg A, Johnsen H, Mikkelsen G, Hov GG. Using probit regression to disclose the analytical performance of qualitative and semi-quantitative tests. Scand J Clin Lab Invest. 2016; 76:515–519. PMID: 27385434.
7. CLSI. Using proficiency testing and alternative assessment to improve medical laboratory quality. 3rd ed. CLSI QMS24. Wayne, PA: Clinical and Laboratory Standards Institute;2016.
8. United States Food and Drug Administration. Guidance for industry and FDA staff–review criteria for assessment of qualitative fecal occult blood in vitro diagnostic devices. Updated on Aug 2007. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfggp/Results.CFM?Doc_Type=1&Doc_IsCur=1&Doc_OFFICE=OIVD&lookandfeel=1&SORT_ORDER=origin,documentdate%20desc.
9. Segnan N, Patnick J, editors. European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st ed. Luxembourg: Publications Office of the European Union;2010. p. 103–144.
10. Korean Ministry of Food and Drug Safety. Guideline to establish performance evaluation for fecal occult blood tests. Updated on Feb 2018. http://www.nifds.go.kr/brd/m_15/view.do?seq=11937&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=14.
Table 1
Estimated cutoff concentration of pre/post-improvement qualitative FITs




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download