Dear Editor,
Fluoranthene (FR) is a polycyclic aromatic hydrocarbon (PAH), a compound that acts as an environmental pollutant. However, no study has examined the carcinogenicity, hematopoietic stem cell (HSC) toxicity, or global hematotoxicity of FR. FR-induced cytotoxicity could be mediated through the cytoplasmic aryl hydrocarbon receptor (AhR), which is a ligand-dependent transcription factor that is activated by various environmental pollutants, including PAHs [12]. AhR is highly expressed in hematopoietic stem and progenitor cells (HSPCs), and its inhibition leads to a marked expansion of human umbilical cord blood-derived HSPCs following cytokine stimulation [3]. Importantly, a recent study has suggested that AhR plays a critical role in the growth and differentiation of HSCs [4], but there has been limited data regarding the effect of AhR antagonist on HSC differentiation [3]. Moreover, whether AhR plays an earlier role in human hematopoietic development remains unknown [3]. We hypothesized that blocking AhR using its antagonist might be useful in preventing FR-related hematotoxicity. Therefore, we initially investigated FR-induced HSC toxicity and the possible preventive effect of AhR antagonist on FR-induced hematotoxicity. Additionally, we examined the direct effect of AhR antagonist on HSC self-renewal proliferation, as well as differentiation. This is the first study examining the effect of AhR antagonist on FR-induced toxicity.
Our study was approved by the Institutional Review Board (IRB) of Chonnam National University Hwasun Hospital, Hwasun, Korea (IRB No. 2009-35), and all samples were collected after obtaining written informed consent.
HSCs were isolated using a peripheral blood stem cell (PBSC) collection procedure following recruitment of four healthy donors and one lymphoma patient with an autologous HSC transplantation setting. Briefly, granulocyte colony-stimulating factor (G-CSF) was administered continuously until the white blood cell count reached a threshold above 1×109/L. The patient underwent leukapheresis once or twice using a blood cell separator. To quantify production of PBSCs from each leukapheresis, flow cytometry was used to enumerate and quantify CD34+ cells. This study was performed immediately after PBSC collection. Isolated HSCs were cultured using a methylcellulose medium for 14–16 days at 37℃, in 5% CO2 with 95% humidity to determine the differentiation capacity of primary HSCs. FR (99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock FR solutions were made in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a concentration of 200 M. Colony forming unit-granulocyte and macrophage (CFU-GM) and burst-forming unit-erythroid (BFU-E) were identified and counted using an inverted microscope: CFU-GM showed white colonies, and BFU-E showed faint red colonies. The direct effect of FR on the peripheral blood-HSC (PB-HSC) fraction was a general reduction in the number of myeloid (CFU-GM) and erythroid (BFU-E) colonies; the number of BFU-E colonies was lower than that of CFU-GM colonies.
Next, CH223191 (CH), SR1, UM171, D03, E03, and Hit 90186 were selected as candidate AhR antagonists for a preliminary experiment based on a screening process conducted by a collaborating research laboratory (Zebra Fish Laboratory, Hwasun, Korea) and then synthesized by the Korea Chemical Bank (Daejeon, Korea) (www.chembank.org). To determine the self-renewal capacity of the PBSC fraction isolated from allogeneic donors and an autologous patient, six AhR antagonists were applied in a suspension culture system for HSC expansion. The suspension culture of the PBSC fraction was performed as follows: briefly, PB-derived HSC samples were placed in separate wells of six-well plates and cultured in a serum-free medium containing 100 ng/mL of stem cell factor, 100 ng/mL of fms-like tyrosine kinase 3, 100 ng/mL of thrombopoietin, and 50 ng/mL of G-CSF to examine the self-renewal capacity of the HSC fraction. All tested AhR antagonists resulted in a remarkable increase of the PB-HSC fraction in a time- and dose-dependent manner in both the autologous and allogeneic PB-HSC fractions. SR1 and E03 resulted in the greatest increase in self-renewal proliferation in the autologous HSC fraction compared with other AhR antagonists. Additionally, UM171 and Hit 90186 had the strongest effect on HSC expansion in the allogeneic HSC fraction (Fig. 1).
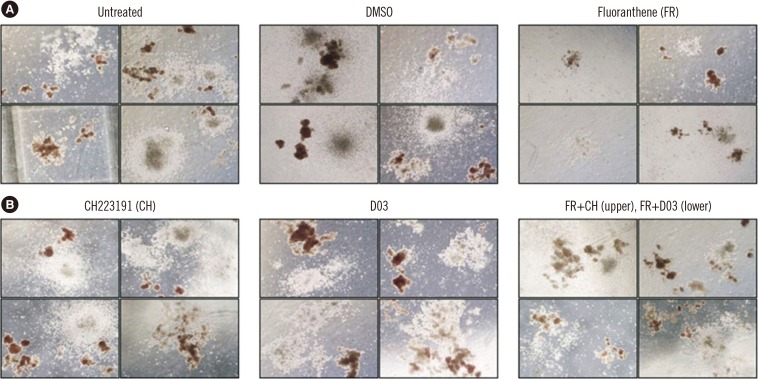
CH and D03 were selected based on the preliminary tests for AhR blockage experiments to examine the preventive effect on FR-induced cytotoxicity during HSC differentiation. CH or D03 were applied together with FR to a methylcellulose culture. The effect of these AhR antagonists on HSC differentiation was examined using a CFU assay. No remarkable effect on HSC differentiation was observed following treatment of the PB-HSC fraction with these AhR antagonists. However, we confirmed that there was no significant FR-induced cytotoxic effect on the PB-HSC fraction following combined treatment with AhR antagonists and FR. It seems that co-treatment of CH and D03 with FR restored cell number while repairing cell morphology. These findings indicate that AhR antagonists have the potential to protect against FR-induced cytotoxicity (Fig. 2).
This study used descriptive analysis or presentation rather than the statistical analysis because of limited number of primary PBSC samples. Practically, it was not easy to gather a large number of PBSC samples due to relatively small PBSC volume. Moreover, our data were collected from a single center in Korea, so the findings may not be generalized to other institutions. The limited number of patients and PBSC samples might preclude definitive conclusions on the effect of FR and AhR antagonist on HSC.
In conclusion, the CFU of the HSC lineage, including erythroid and granulocytic progenitor cells, was remarkably suppressed following FR exposure. Our results indicate that BFU-E was more reduced than CFU-GM. These results seem to be related to anemia, which requires further research. Moreover, AhR antagonists might prevent FR-induced hematotoxicity effectively.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2018R1D1A1B07040984), and grants from the Environmental Health Center through the Ministry of Environment, Korea.
References
1. Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell-cell contact and tumor growth. Carcinogenesis. 2010; 31:1319–1328. PMID: 20106901.
2. Rondelli CM, Larsen MC, N'Jai A, Czuprynski CJ, Jefcoate CR. PAHs target hematopoietic linages in bone marrow through Cyp1b1 primarily in mesenchymal stromal cells but not AhR: A reconstituted in vitro model. Stem Cells Int. 2016; 2016:1753491. PMID: 27891153.
3. Angelos MG, Ruh PN, Webber BR, Blum RH, Ryan CD, Bendzick L, et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood. 2017; 129:3428–3439. PMID: 28533309.
4. Smith BW, Rozelle SS, Leung A, Ubellacker J, Parks A, Nah SK, et al. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood. 2013; 122:376–385. PMID: 23723449.
Fig. 1
Time- and dose-dependent effect of AhR antagonist treatment on HSC self-renewal proliferation in suspension cultures from autologous and allogeneic PB-HSC fractions. (A) Effect of AhR antagonist on the self-renewal of the autologous HSC fraction. SR1 and E03 resulted in the greatest increase in self-renewal proliferation in the autologous PB-HSC faction compared with other AhR antagonists. (B) Effect of AhR antagonist on the self-renewal of the allogeneic HSC fraction. UM and HIT resulted in the greatest increase in self-renewal proliferation in the allogeneic PB-HSC faction compared with other AhR antagonists.
Abbreviations: UN, untreated; DMSO, dimethyl sulfoxide; HSC, hematopoietic stem cell; PB-HSC, peripheral blood-derived hematopoietic stem cell; AhR, aryl hydrocarbon receptor; UM, UM171; HIT, Hit 90186.
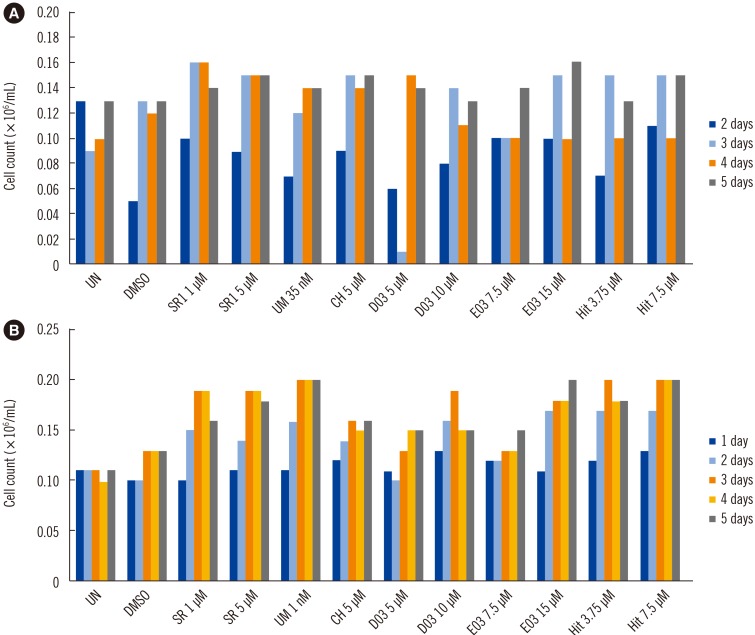
Fig. 2
Effect of the AhR antagonists on HSC differentiation using a colony forming unit (CFU) assay for PBSC. (A) Exposure of the PBSC fraction to FR induced remarkable suppression of CFC formation. (B) Treatment of the PB-HSC fraction with AhR antagonists (CH and D03) did not show a remarkable effect on HSC differentiation, whereas co-treatment of CH and D03 with FR restored cell number, as well as reconstructed cell morphology. Thus, AhR antagonists seem to have some restorative effect on PBSC following FR exposure.
Abbreviations: CFC, colony-forming cell; DMSO, dimethyl sulfoxide; HSC, hematopoietic stem cell; PB-HSC, peripheral blood-derived hematopoietic stem cell; PBSC, peripheral blood-derived stem cell; AhR, aryl hydrocarbon receptor; FR, fluoranthene.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download