Accurate assessment of HLA antibody is crucial for successful transplant management [
1]. The Luminex-based single antigen bead (SAB) assay is widely used for sensitive detection of low concentrations of antibody [
2345]. In the SAB assay, antibody amount is determined semi-quantitatively and is expressed as the mean fluorescence intensity (MFI) after reaction with anti-human IgG antibody reagent labeled with a fluorescent dye. MFI values correlate with transplant-related outcomes; thus, monitoring MFI values may be useful for risk assessment after transplantation [
67]. In addition, SAB assays for detecting C1q-binding HLA antibodies (C1q SAB) have been introduced to better discriminate clinically relevant antibodies [
8].
However, clinical application of the SAB assay is limited because of technical issues, including assay variability, complement interference, and the prozone effect [
9]. Inhibition causing false-negative or falsely decreased MFI values has been observed in approximately 70% of highly sensitized patients [
10]. Several studies have attempted to eliminate the prozone effect by treatment with EDTA or dithiothreitol (DTT), preheating, and/or dilution of serum [
91112]. There are multiple explanations for the prozone effect, including bead saturation [
1314], steric hindrance from complement complex [
11], and the presence of IgM HLA-specific antibodies [
15]. Complement interference involves covalent binding and accumulation of C4 and C3 degradation products on the immune complex, which prevent fluorescent anti-IgG from binding to IgG. EDTA eliminates the prozone effect because, as an iron-chelating reagent, it can inhibit the formation of C1qrs complement complex and prevent complement cascade activation [
16]. DTT disrupts the pentameric structure of IgM and the disulfide bonds in complement molecules, thus reducing the prozone effect [
15]. In this respect, SAB assays as a standard should include serum treatment to correct the prozone effect because MFI values are often used as a surrogate marker of antibody strength. Although a recent meeting report recommended EDTA and/or titration [
1], there is currently no verified standard method, and different treatment methods are used across different laboratories.
We compared MFI values of SAB assays in serum samples treated with or without EDTA, DTT, or dilution. In addition, we evaluated the correlation between IgG-MFI and C1q-MFI values after EDTA treatment. To our knowledge, this is the first study to investigate the effects of EDTA on specific HLA loci in combination with C1q-MFI.
We collected leftover sera from 27 highly sensitized patients (median age=49 years, M:F=14:13). All samples contained strong HLA antibodies (MFI≥10,000), and the median panel-reactive antibody (%) was 95%. The patients had become sensitized by previous transplantation with or without transfusion or pregnancy (81.5%, N=22), previous pregnancy and transfusion (7.4%, N=2), previous pregnancy alone (7.4%, N=2), or previous transfusion alone (3.7%, N=1). The Institutional Review Board of Seoul St. Mary's Hospital, Seoul, Korea, approved this study (KC18SESI0323). LABScreen Single Antigen kits and the LABScan 3D system (One Lambda, A Thermo Fisher Scientific Brand, Canoga Park, CA, USA) were used to detect HLA antibodies (11 samples for class I only, eight samples for class II only, and eight samples for both class I and class II). For EDTA treatment, a 0.5 M EDTA solution was diluted 1:20 (final concentration of 25 mM), added to the serum, vortexed, and centrifuged at room temperature for 10 minutes before testing [
10]. We defined the prozone effect as doubled MFI values, with an MFI cutoff of 1,000, or 5,000 MFI increment after serum treatment compared with the results from naïve serum, considering recent recommendations and reports [
11217].
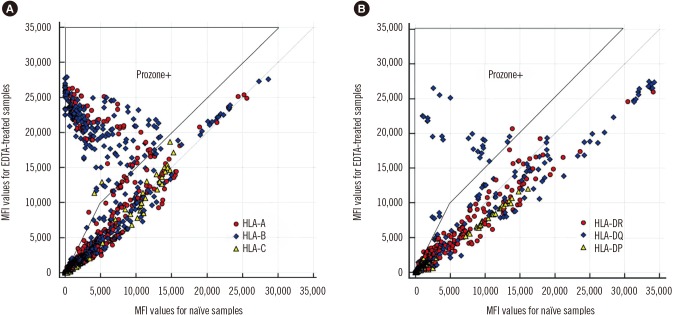
In a comparison of naïve and EDTA-treated samples, we investigated MFI data from 3,363 beads (1,843 class I and 1,520 class II) from 35 tests (19 class I and 16 class II) (
Fig. 1). The prozone effect was identified in 53% (10 out of 19) of class I antibody tests and in 31% (5 out of 16) of class II antibody tests after EDTA treatment. Of all beads, 8.3% (26.5% of positive beads) were affected by complement inhibition, with the median MFI increasing from 2,921 (range, 0–13,890) to 21,043 (1,024–27,874) after EDTA treatment. Regarding HLA specificity, prozone effect incidences were as follows: HLA-A, 28.1%; HLA-B, 43.6%; HLA-C, 3.6%; HLA-DR, 2.7%; HLA-DQ, 18.0%; and HLA-DP, <0.1% of positive beads. These findings were consistent with a previous report wherein HLA-C beads were the least affected among class I beads and HLA-DQ beads were the most affected among class II beads [
18].
Fig. 1
Comparison of MFI values in naïve and EDTA-treated sera. (A) Class I IgG-SAB results in 19 sera (1,843 beads); (B) Class II IgG-SAB results in 16 sera (1,520 beads).
Abbreviations: MFI, mean fluorescence intensity; SAB, single antigen bead.

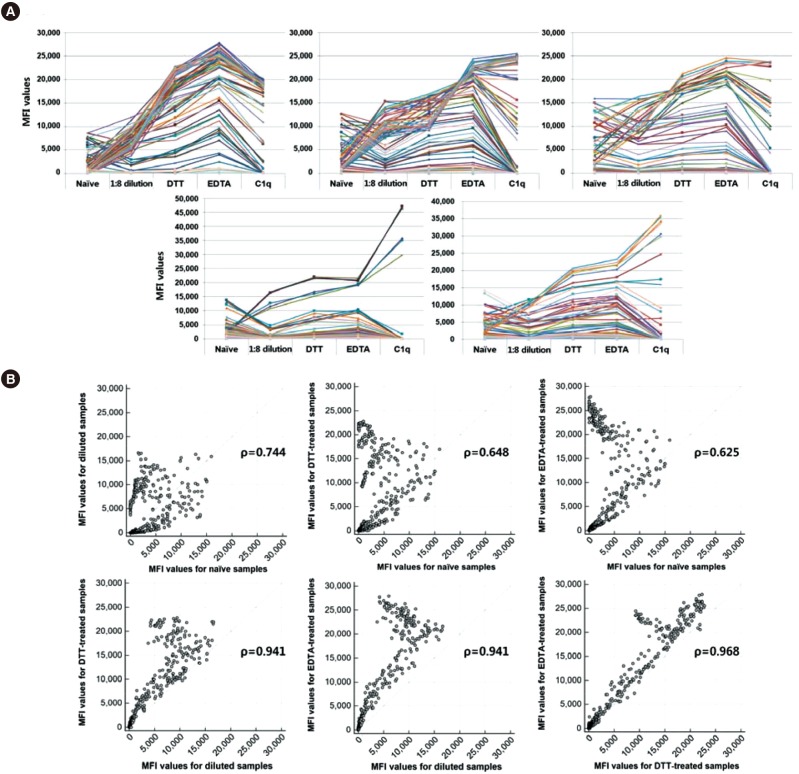
We selected five sera and additionally tested class I IgG after 1:8 dilution in phosphate-buffered saline and treatment with 5 mM DTT at 37℃ for 30 minutes [
19]. When we compared the three serum treatment methods, EDTA treatment was the most efficacious for overcoming prozone effect (
Fig. 2). This finding was consistent with previous studies that showed the superior efficacy of EDTA for overcoming the prozone effect [
1216]. DTT treatment and dilution resulted in small increases in MFI values in some of the beads, indicating a residual prozone effect, presumably because only IgM antibodies were removed (
Supplemental Data Table S1).
Fig. 2
MFI values for naïve samples and samples treated with 1:8 dilution, DTT, and EDTA, and C1q assay using sera from five sensitized patients. (A) MFI values for all samples with various treatments. (B) Correlations between MFI values for naïve, diluted, DTT-treated, and EDTA-treated samples.
Abbreviations: MFI, mean fluorescence intensity; DTT, dithiothreitol.

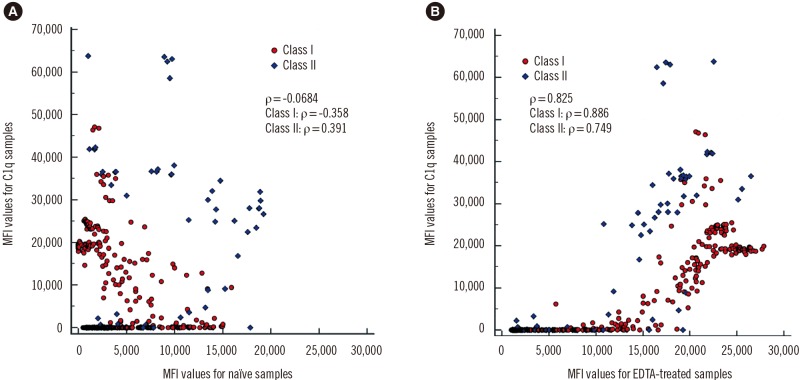
We subjected 10 samples with the prozone effect (N=5 for class I and N=5 for class II HLA antibodies) to C1qScreen assays (One Lambda). We selected beads that were positive in EDTA-treated sera to calculate Spearman's correlation coefficient (ρ). MFI values were summarized as median (range). For statistical analysis, we used MedCalc Statistical Software version 18.9 (MedCalc Software, Ostend, Belgium).
When naïve sera were used, the correlation between IgG-MFI values and C1q-MFI values was weak (ρ=−0.0684; class I: −0.358, class II: 0.391). After EDTA treatment, the correlation between IgG-MFI values and C1q-MFI values became stronger (ρ=0.825; class I: 0.886, class II: 0.749) (
Fig. 3). Beads that displayed low IgG-MFI values in naïve sera, but high C1q-MFI values, showed high IgG-MFI values after EDTA treatment. In naïve sera, C1q-SAB beads with MFI≥1,000 (19.7% of all beads) had a median IgG-MFI value of 2,842 (range, 0–19,256). In EDTA-treated sera, C1q-positive beads had a median IgG-MFI value of 20,900 (1,539–27,874). These results supported previous findings that C1q binding ability depends on the concentration of HLA antibody [
12]. Tambur and Wiebe [
9] reported that MFI cutoffs that predicted C1q positivity varied from 6,237 to 14,154 using non-treated samples. However, not all beads that had high IgG-MFI values in EDTA-treated sera showed C1q positivity, and some high-level IgG antibodies did not bind to C1q. When we plotted IgG-MFI against C1q-MFI values from the data in 10 patients separately, we found a wide range of correlation coefficients (0.283–0.977). Therefore, it remains to be elucidated whether there is an interdependence between C1q positivity and IgG-MFI values.
Fig. 3
Correlation of MFI values from C1q- and IgG-SAB assays using (A) naïve sera and (B) EDTA-treated sera.
Abbreviations: MFI, mean fluorescence intensity; SAB, single antigen bead.

Some limitations of the present study were that we could not test serial dilutions of samples, and we did not confirm the HLA-specific antibodies against intact HLA antigen on the lymphocyte. Because high-level antibodies against denatured HLA protein might also bind to C1q [
20], future studies on interference by denatured HLA are needed. In addition, we selected samples with high panel-reactive antibody and strong HLA antibodies. Thus, our results may not represent the frequency of the prozone effect in SAB assays in general.
In conclusion, we confirmed the efficacy of EDTA treatment for eliminating the prozone effect. Further, EDTA treatment had a positive effect on the correlation between IgG MFI and C1q MFI values. Our study highlights the importance of method standardization to correct the prozone effect.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download