1. Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood. 1987; 69:454–459. PMID:
3492222.
2. Goodeve AC. The genetic basis of von Willebrand disease. Blood Rev. 2010; 24:123–134. PMID:
20409624.
3. Mancuso DJ, Tuley EA, Westfield LA, Worrall NK, Shelton-Inloes BB, Sorace JM, et al. Structure of the gene for human von Willebrand factor. J Biol Chem. 1989; 264:19514–19527. PMID:
2584182.
4. Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA. Sequence and structure relationships within von Willebrand factor. Blood. 2012; 120:449–458. PMID:
22490677.
5. Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006; 4:2103–2114. PMID:
16889557.
6. Schneppenheim R. The pathophysiology of von Willebrand disease: therapeutic implications. Thromb Res. 2011; 128(S1):S3–S7. PMID:
22221847.
7. Baronciani L, Goodeve A, Peyvandi F. Molecular diagnosis of von Willebrand disease. Haemophilia. 2017; 23:188–197. PMID:
28220580.
8. Kim HJ, Chung HS, Kim SK, Yoo KY, Jung SY, Park IA, et al. Mutation spectrum and inhibitor risk in 100 Korean patients with severe haemophilia A. Haemophilia. 2012; 18:1008–1013. PMID:
22741565.
9. Yadegari H, Driesen J, Hass M, Budde U, Pavlova A, Oldenburg J. Large deletions identified in patients with von Willebrand disease using multiple ligation-dependent probe amplification. J Thromb Haemost. 2011; 9:1083–1086. PMID:
21410641.
10. Wang QY, Song J, Gibbs RA, Boerwinkle E, Dong JF, Yu FL. Characterizing polymorphisms and allelic diversity of von Willebrand factor gene in the 1000 Genomes. J Thromb Haemost. 2013; 11:261–269. PMID:
23216583.
11. Song J, Choi JR, Song KS. Investigation of von Willebrand factor gene mutations in Korean von Willebrand disease patients. Korean J Lab Med. 2007; 27:169–176. PMID:
18094571.
12. Rodeghiero F, Castaman G, Tosetto A, Batlle J, Baudo F, Cappelletti A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005; 3:2619–2626. PMID:
16359502.
13. Corrales I, Ramírez L, Altisent C, Parra R, Vidal F. Rapid molecular diagnosis of von Willebrand disease by direct sequencing. Detection of 12 novel putative mutations in VWF gene. Thromb Haemost. 2009; 101:570–576. PMID:
19277422.
14. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum Mutat. 2016; 37:564–569. PMID:
26931183.
15. Cabrera N, Casaña P, Cid AR, Moret A, Moreno M, Palomo A, et al. First application of MLPA method in severe von Willebrand disease. Confirmation of a new large VWF gene deletion and identification of heterozygous carriers. Br J Haematol. 2011; 152:240–242. PMID:
20955405.
16. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID:
25741868.
17. Mikhail S, Aldin ES, Streiff M, Zeidan A. An update on type 2B von Willebrand disease. Expert Rev Hematol. 2014; 7:217–231. PMID:
24521271.
18. Yadegari H, Driesen J, Pavlova A, Biswas A, Hertfelder HJ, Oldenburg J. Mutation distribution in the von Willebrand factor gene related to the different von Willebrand disease (VWD) types in a cohort of VWD patients. Thromb Haemost. 2012; 108:662–671. PMID:
22871923.
19. Eikenboom J, Van Marion V, Putter H, Goodeve A, Rodeghiero F, Castaman G, et al. Linkage analysis in families diagnosed with type 1 von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 VWD. J Thromb Haemost. 2006; 4:774–782. PMID:
16634746.
20. James PD, Paterson AD, Notley C, Cameron C, Hegadorn C, Tinlin S, et al. Genetic linkage and association analysis in type 1 von Willebrand disease: results from the Canadian type 1 VWD study. J Thromb Haemost. 2006; 4:783–792. PMID:
16634747.
21. Meyer D, Fressinaud E, Gaucher C, Lavergne JM, Hilbert L, Ribba AS, et al. Gene defects in 150 unrelated French cases with type 2 von Willebrand disease: from the patient to the gene. INSERM Network on Molecular Abnormalities in von Willebrand disease. Thromb Haemost. 1997; 78:451–456. PMID:
9198195.
22. Casonato A, Pontara E, Sartorello F, Cattini MG, Perutelli P, Bertomoro A, et al. Identifying carriers of type 2N von Willebrand disease: procedures and significance. Clin Appl Thromb Hemost. 2007; 13:194–200. PMID:
17456630.
23. Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von Willebrand factor. J Biol Chem. 2007; 282:35604–35611. PMID:
17925407.
24. Shapiro SE, Nowak AA, Wooding C, Birdsey G, Laffan MA, McKinnon TA. The von Willebrand factor predicted unpaired cysteines are essential for secretion. J Thromb Haemost. 2014; 12:246–254. PMID:
24283831.
25. Eikenboom JC, Matsushita T, Reitsma PH, Tuley EA, Castaman G, Briët E, et al. Dominant type 1 von Willebrand disease caused by mutated cysteine residues in the D3 domain of von Willebrand factor. Blood. 1996; 88:2433–2441. PMID:
8839833.
26. Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand disease (MCMDM-1VWD). Blood. 2007; 109:112–121. PMID:
16985174.
27. Eikenboom JC, Castaman G, Vos HL, Bertina RM, Rodeghiero F. Characterization of the genetic defects in recessive type 1 and type 3 von Willebrand disease patients of Italian origin. Thromb Haemost. 1998; 79:709–717. PMID:
9569178.
28. Tjernberg P, Vos HL, Castaman G, Bertina RM, Eikenboom JC. Dimerization and multimerization defects of von Willebrand factor due to mutated cysteine residues. J Thromb Haemost. 2004; 2:257–265. PMID:
14995987.
29. Casari C, Lenting PJ, Wohner N, Christophe OD, Denis CV. Clearance of von Willebrand factor. J Thromb Haemost. 2013; 11(S1):202–211. PMID:
23809124.
30. Choung HS, Kim HJ, Gwak GY, Kim SH, Kim DK. Inherited protein S deficiency as a result of a large duplication mutation of the
PROS1 gene detected by multiplex ligation-dependent probe amplification. J Thromb Haemost. 2008; 6:1430–1432. PMID:
18489710.
31. Kim HJ, Kim DK, Yoo KY, You CW, Yoo JH, Lee KO, et al. Heterogeneous lengths of copy number mutations in human coagulopathy revealed by genome-wide high-density SNP array. Haematologica. 2012; 97:304–309. PMID:
21993689.
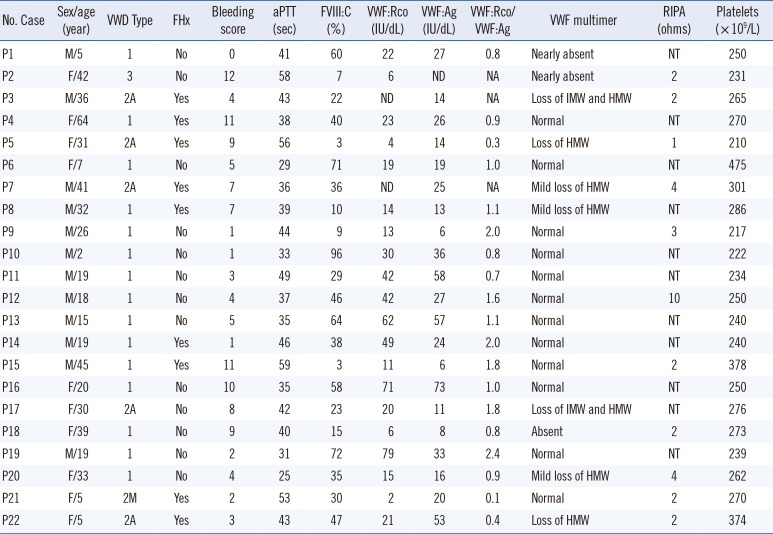
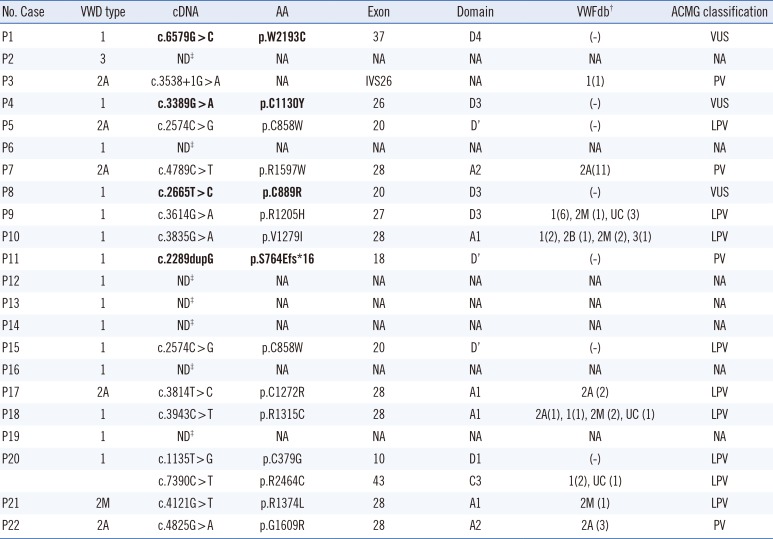
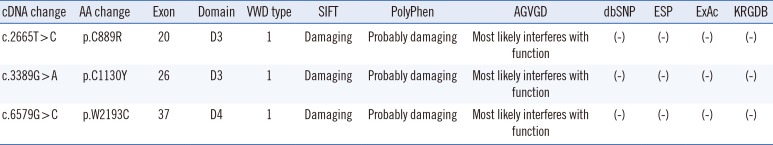




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download