1. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33:676–682. PMID:
20190296.
2. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U. Hyperglycemia and adverse pegnancyoutcomes. N Engl J Med. 2008; 358:1991–2002. PMID:
18463375.
3. Farrar D, Duley L, Medley N, Lawlor DA. Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst Rev. 2015; 1:CD007122. PMID:
25604891.
4. American Diabetes Association. Management of diabetes in pregnancy: standards of medical care in diabetes - 2019. Diabetes Care. 2019; 42(Suppl 1):S165–S172. PMID:
30559240.
5. Committee on Practice Bulletins--Obstetrics.Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013; 122:406–416. PMID:
23969827.
6. Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden DM. Screening tests for gestational diabetes: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013; 159:115–122. PMID:
23712349.
7. Bonongwe P, Lindow SW, Coetzee EJ. Reproducibility of a 75G oral glucose tolerance test in pregnant women. J Perinat Med. 2015; 43:333–338. PMID:
25405716.
8. Maesa JM, Fernández-Riejos P, Mora CS, de Toro M, Valladares PM, González-Rodriguez C. Evaluation of Bio-Rad D-100 HbA1c analyzer against Tosoh G8 and Menarini HA-8180V. Pract Lab Med. 2016; 5:57–64. PMID:
28856205.
9. WHO. Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011. p. 1–25.
10. International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334. PMID:
19502545.
11. Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and adverse pregnancy outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012; 35:574–580. PMID:
22301123.
12. Maesa JM, Fernandez-Riejos P, Sanchez-Mora C, Toro-Crespo M, Gonzalez-Rodriguez C. Application of six sigma model to evaluate the analytical quality of four HbA1c analyzers. Clin Lab. 2017; 63:79–83. PMID:
28164511.
13. GrupoEspañol Diabetes y Embarazo. Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada en 2014. Diabetologia. 2015; 31:45–59.
14. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979; 28:1039–1057. PMID:
510803.
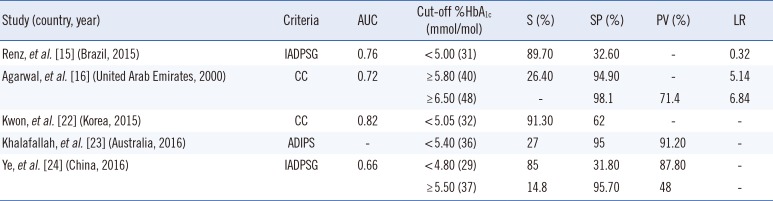
15. Renz PB, Cavagnolli G, Weinert LS, Silveiro SP, Camargo JL. HbA1c test as a tool in the diagnosis of gestational diabetes mellitus. PLoS One. 2015; 10:e0135989. PMID:
26292213.
16. Agarwal MM, Hughes PF, Punnose J, Ezimokhai M, Thomas L. Gestational diabetes screening of a multiethnic, high-risk population using glycated proteins. Diabetes Res Clin Pract. 2001; 51:67–73. PMID:
11137184.
18. Benhalima K, Damm P, Van Assche A, Mathieu C, Devlieger R, Mahmood T, et al. Screening for gestational diabetes in Europe: where do we stand and how to move forward?: A scientific paper commissioned by the European Board & College of Obstetrics and Gynaecology (EBCOG). Eur J Obstet Gynecol Reprod Biol. 2016; 201:192–196. PMID:
27105781.
19. National Institute for Health and Care Excellence. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. 2015. p. 2–65.
20. Aceituno Velasco L, Aguado Maldonado J, Arribas Mir L, Caño Aguilar A, Corona Páez I, Martín López J, et al. Procesoasistencialintegradoembarazo, parto y puerperio. 3. Junta Andalucía ConserjeríaIgualdad, Salud u PolíticasSoc;2014. p. 1–73.
21. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982; 144:768–773. PMID:
7148898.
22. Kwon SS, Kwon JY, Park YW, Kim YH, Lim JB. HbA1c for diagnosis and prognosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2015; 110:38–43. PMID:
26344325.
23. Khalafallah A, Phuah E, Al-Barazan AM, Nikakis I, Radford A, Clarkson W, et al. Glycosylated haemoglobin for screening and diagnosis of gestational diabetes mellitus. BMJ Open. 2016; 6:e011059.
24. Ye M, Liu Y, Cao X, Yao F, Liu B, Li Y, et al. The utility of HbA1c for screening gestational diabetes mellitus and its relationship with adverse pregnancy outcomes. Diabetes Res Clin Pract. 2016; 114:43–49. PMID:
27103368.
25. Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Honghua HJ, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care. 2013; 36:2931–2936. PMID:
23757434.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download