This article has been
cited by other articles in ScienceCentral.
Abstract
Tea is the most widely consumed beverage after water in the world. The consumption of iced tea has increased in Western countries and spiked among teenagers for enjoyment, freshening up and alertness. A teenager presented with symptoms of hepatitis. Liver ultrasound revealed sludge in the gallbladder. Laboratory investigations excluded all known causes of hepatotoxicity. Detail nutritional history revealed that the patient had been drinking 1.5–2 liters of black iced tea per day for the last three months. He was immediately advised to stop drinking any tea. Gradually all symptoms disappeared and two months after discontinuation of the tea, all liver enzymes returned to normal and the sludge in the gallbladder disappeared. This case report underlines the importance of a meticulous assessment of a child's dietary behavior when investigating a case of hepatotoxicity and raises awareness about the potential side effects of tea overconsumption.
Keywords: Tea, Chemical and drug induced liver injury, Teenagers
INTRODUCTION
After water, tea (the green or black variety), is the second most widely consumed beverage in the world. The consumption of iced tea has increased in many countries, and it often overtakes the consumption of traditional hot tea, particularly among adolescents [
1]. Additionally, the consumption of iced tea has recently increased among teenagers for enjoyment, as a refreshment, and for alertness [
1].
Both green and black tea varieties are produced from the leaves of the
Camellia sinensis plant. Green tea is produced when tea leaves are dried and steamed after harvesting. Black tea is produced by the oxidization of tea leaves, which results in the formation of theaflavins and thearubigins that give black tea its color [
2]. Consumption of excessive quantities of green (but not black tea) is associated with hepatotoxicity [
3].
To our knowledge, to date, no published article in the available literature has described liver damage secondary to the consumption of high quantities of iced black tea in adolescents. This case report underlines the importance of assessing an adolescent's dietary behavior when investigating hepatotoxicity. Moreover, health care providers, parents, and teenagers should be aware of the potential adverse effects of excessive tea consumption.
CASE REPORT
A previously healthy male adolescent aged 12 years and 6 months presented with a 2-week history of pain in the right upper abdomen and a 1-day history of itching prior to presentation. He also reported that his urine was dark colored. He denied other symptoms such as fever, nausea, vomiting, or bleeding.
His medical history was negative for blood transfusion, exposure to hepatotoxic drugs, recent overseas travel, signs of viral infection, allergies, atopy, or asthma. He denied any alcohol or illicit drug use. His family history was unknown, as he is an adopted child. He had been vaccinated against viral hepatitis A and B.
Physical examination revealed that the teenager was in good general condition. His height (152 cm), weight (43.2 kg), and body mass index (18.7 kg/m2) were at the 50th percentile for males. He was afebrile without any signs of jaundice, and his vital signs were normal. The abdomen was soft with normal bowel sounds. He showed mild tenderness to deep palpation of the right upper abdominal quadrant.
Upon further questioning, the patient reported consuming 1.5–2 liters per day of iced black tea over 3 months prior to presentation. The product primarily contained the following ingredients: sugar (4.5 g/100 mL) (sugar, fructose), black tea extract (0.09%), citric acid, apricot juice (0.1%), and water. He was advised to discontinue drinking any kind of tea. Initial laboratory tests showed elevated levels of liver enzymes, γ-glutamyl transpeptidase, alkaline phosphatase, and bilirubin. However, renal function and coagulation tests, as well as serum creatine kinase and electrolyte levels were within normal limits (
Table 1). The prothrombin time was 11.8 seconds (10–13), the international normalized ratio; 1.05 (0.85–1.25); and the ammonia level, 45 μg/dL (31–123).
Table 1
Initial and subsequent relevant to the disease laboratory results

|
Parameters (normal range) |
4 months prior |
Day 1 |
Day 7 |
Day 15 |
Day 30 |
Day 60 |
|
ALT (3–41 U/L) |
19 |
427 |
215 |
105 |
88 |
17 |
|
AST (3–38 U/L) |
25 |
402 |
202 |
107 |
70 |
27 |
|
ALP (30–120 U/L) |
96 |
900 |
746 |
636 |
345 |
100 |
|
γ-glutamyl transpeptidase (9–55 U/L) |
47 |
102 |
87 |
77 |
52 |
45 |
|
Total bilirubin (0.3–1.2 mg/dL) |
0.8 |
3 |
1.5 |
1.01 |
1 |
0.9 |
|
Direct bilirubin (0.10–0.20 mg/dL) |
0.1 |
1.8 |
1 |
0.5 |
0.15 |
0.1 |

An abdominal ultrasound scan showed sludge in the gallbladder without increased echogenicity of the liver (
Fig. 1).
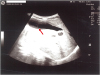 | Fig. 1 Abdominal ultrasound scan showing sludge in the gallbladder.
|
The differential diagnosis included chronic viral hepatitis (hepatitis B, C, D), acute viral hepatitis (Epstein-Barr virus [EBV], cytomegalovirus [CMV], herpes simplex virus 1 [HSV1], human immunodeficiency virus [HIV], and enterovirus), nonalcoholic fatty liver disease, hemochromatosis, autoimmune hepatitis, Wilson disease, alpha-1 antitrypsin deficiency, alcoholic liver disease, muscle disorders (e.g., subclinical inborn errors of muscle metabolism), use of herbal medications, thyroid disorders, and celiac disease.
Laboratory workup to determine the etiology of acute hepatitis including a viral hepatitis panel, CMV/EBV polymerase chain reaction tests, and immunoglobulin-M antibodies, serum ceruloplasmin, copper, iron panel, alpha-1 antitrypsin genotype, HIV, HSV, anti-nuclear antibody, anti-smooth muscle antibody, and anti-liver-kidney microsome antibody showed normal results. Autoimmune cholangitis or an overlap syndrome was ruled out (the antinuclear antibody test result was negative, and immunoglobulin tests, and tests to assess synthetic liver function were within normal limits). Extensive toxicology screening showed negative results, without any indications of acetaminophen toxicity. Screening tests for celiac disease revealed negative results, and thyroid hormone levels were within normal limits. Liver biopsy was not performed because both, the patient and his parents were reluctant to have the test performed.
The patient was asymptomatic, and his physical examination revealed normal findings at his follow-up visit 2 months after discontinuing iced tea consumption. An abdominal ultrasound scan performed at the time revealed no abnormalities.
Subsequent laboratory tests revealed gradual normalization of liver enzymes over the course of 2 months after his initial presentation (
Table 1).
DISCUSSION
Black tea has gained considerable attention for its proposed health benefits [
4] that have been partly attributed to black tea polyphenols, as reported by epidemiological and clinical studies [
5]. Notably, polyphenols are known to show antioxidant, antimutagenic, anticancer, antipathogenic, anti-inflammatory, and anticlastogenic effects [
6]. Reportedly, black tea confers cardioprotective benefits that are attributed to the antioxidative, antithrombogenic, anti-inflammatory, hypotensive, and hypocholesterolemic properties of tea polyphenols [
7]. Interestingly a hepatoprotective effect of black tea has been reported in rats, supporting the beneficial effect of black tea consumption [
8].
The liver is the principal organ for drug metabolism and therefore plays an important role in drug-related pathologies. Several herbal supplements, including green tea extract have been shown to be associated with hepatic damage manifesting as hepatitis, cholestasis, drug-induced autoimmunity, vascular lesions, hepatic failure, and cirrhosis [
39].
The key features used to diagnose herbal and dietary supplement-induced liver injury are similar to the features used to diagnose liver injury caused by other drug products and include the following [
10].
• Exposure to the possible causative agent must precede the onset of liver injury.
• Any underlying liver disease should be ruled out.
• Liver injury may improve when the suspected causative agent is withdrawn (It should be noted that liver injury may initially worsen for days or weeks in a few cases. Moreover, in cases of fulminant disease, decreasing serum levels of liver enzymes may indicate deterioration in liver function).
In the present case, we attributed hepatotoxicity to the consumption of black tea because all other causes of hepatic damage were excluded. The factors that supported our conclusion are that the gallbladder sludge disappeared and liver enzymes returned to normal levels 2 months after discontinuation of the excessive consumption of black iced tea (
Table 1). Additionally, routine check-up performed 4 months before his initial presentation had revealed that the patient's liver enzymes were within normal limits. The Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scale was used, and the patient's score (6) suggested that liver damage in this case was perhaps secondary to tea consumption.
The time between the first day of overconsumption of tea and the onset of liver injury was 5 to 90 days (2 points). The serum alkaline phosphatase level decreased by >50% from its peak value within 180 days (2 points). All causes in groups I and II of the RUCAM scale were ruled out (2 points).
The National Institutes of Health has established a searchable database of herbal medications, dietary supplements, and drugs associated with hepatotoxicity [
11]. A search of all available databases did not reveal any reports describing possible hepatotoxicity secondary to the use of black tea extract, although several cases of green tea-induced hepatotoxicity have been reported [
2312].
Hepatotoxicity secondary to the increased consumption of herbal products may easily be overlooked owing to the following reasons.
• Patients usually consider herbal products to be completely free of adverse effects and may not even mention the consumption of these products to their health care provider [13].
• Physicians may not be aware of the potential hepatotoxicity of herbal products.
The mechanism of green tea-induced hepatotoxicity is unclear. The components responsible for hepatotoxicity are probably catechins and their gallic acid esters, primarily epigallocatechin-3-gallate, which, under certain circumstances, such as in patients who are fasting, may result in reactive oxygen species formation and influence mitochondrial membrane potential [
312]. Consumption of excessively high quantities of hot black tea is not popular among adolescents; however, they may consume excessive amounts of iced tea, as was observed in our patient.
Pediatricians should be aware of the potential hepatotoxicity of herbal and non-herbal tea preparations. A detailed dietary history should not be overlooked in cases of hepatotoxicity. Herbal products may easily be ignored because patients generally consider herbal products healthy and may not even mention their consumption.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download