1. Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009; 48:1290–1295.

2. Samdani AJ, Bana SH, Azam T. Lymphangioma circumscriptum: report of three cases from Saudi Arabia. Int J Dermatol. 2006; 45:840–842.

3. Bauer BS, Kernahan DA, Hugo NE. Lymphangioma circumscriptum--a clinicopathological review. Ann Plast Surg. 1981; 7:318–326.

4. Morris M. Lymphangioma circumscriptum. In : Unna PG, Morris M, Duhring LA, Leloir H, editors. International atlas of rare skin diseases. London: Lewis;1889. p. 1–4.
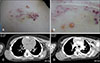
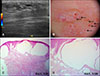
5. Massa AF, Menezes N, Baptista A, Moreira AI, Ferreira EO. Cutaneous Lymphangioma circumscriptum-dermoscopic features. An Bras Dermatol. 2015; 90:262–264.
6. Arpaia N, Cassano N, Vena GA. Dermoscopic features of cutaneous lymphangioma circumscriptum. Dermatol Surg. 2006; 32:852–854.

7. Amini S, Kim NH, Zell DS, Oliviero MC, Rabinovitz HS. Dermoscopic-histopathologic correlation of cutaneous lymphangioma circumscriptum. Arch Dermatol. 2008; 144:1671–1672.

8. Gencoglan G, Inanir I, Ermertcan AT. Hypopyon-like features: new dermoscopic criteria in the differential diagnosis of cutaneous lymphangioma circumscriptum and haemangiomas? J Eur Acad Dermatol Venereol. 2012; 26:1023–1025.

9. Fioramonti P, Maruccia M, Ruggieri M, Onesti MG. A rare case of lymphangioma in the gluteal region: surgical treatment combined with sclerotherapy and laser therapy. Aesthetic Plast Surg. 2013; 37:960–964.

10. Browse NL, Whimster I, Stewart G, Helm CW, Wood JJ. Surgical management of 'lymphangioma circumscriptum'. Br J Surg. 1986; 73:585–588.

11. Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years' experience with lymphangiomas in children. J Pediatr Surg. 1999; 34:1164–1168.

12. Seber S, Köse N, Turgut A, Ozçelik A, Göktürk E. Giant cystic lymphangioma cavernousum of lower limb with overlying lymphangioma circumscriptum. J Pediatr Orthop B. 2004; 13:284–286.

13. Irvine AD, Sweeney L, Corbett JR. Lymphangioma circumscriptum associated with paravesical cystic retroperitoneal lymphangioma. Br J Dermatol. 1996; 134:1135–1137.

14. Rao MV, Thappa DM, Ratnakar C. Lymphangioma circumscriptum of the skin and tongue associated with cystic hygroma. Indian J Otolaryngol Head Neck Surg. 1998; 50:266–268.

15. Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977; 113:24–30.

16. Preeti A, Kumar R. Cystic hygroma: cytological and radiological co-relation. J Clin Diagn Res. 2011; 5:1008–1010.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download