1. Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997; 29:157–166.
2. Grois N, Pötschger U, Prosch H, Minkov M, Arico M, Braier J, et al. Risk factors for diabetes insipidus in langerhans cell histiocytosis. Pediatr Blood Cancer. 2006; 46:228–233.

3. Hartman KS. Histiocytosis X: a review of 114 cases with oral involvement. Oral Surg Oral Med Oral Pathol. 1980; 49:38–54.

4. Ardekian L, Peled M, Rosen D, Rachmiel A, Abu el-Naaj I, Laufer D. Clinical and radiographic features of eosinophilic granuloma in the jaws: review of 41 lesions treated by surgery and low-dose radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87:238–242.

5. Guiglia R, Pizzo G, Aricò M, Maresi E, Compilato D, Campisi G. Bifocal manifestation of eosinophilic granuloma in a pediatric patient. Med Sci Monit. 2009; 15:CS95–CS99.
6. Dagenais M, Pharoah MJ, Sikorski PA. The radiographic characteristics of histiocytosis X. A study of 29 cases that involve the jaws. Oral Surg Oral Med Oral Pathol. 1992; 74:230–236.
7. Kim BE, Koh KN, Suh JK, Im HJ, Song JS, Lee JW, et al. Korea Histiocytosis Working Party. Clinical features and treatment outcomes of Langerhans cell histiocytosis: a nationwide survey from Korea Histiocytosis Working Party. J Pediatr Hematol Oncol. 2014; 36:125–133.

8. Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010; 116:1919–1923.

9. Yasko AW, Fanning CV, Ayala AG, Carrasco CH, Murray JA. Percutaneous techniques for the diagnosis and treatment of localized Langerhans-cell histiocytosis (eosinophilic granuloma of bone). J Bone Joint Surg Am. 1998; 80:219–228.

10. Karagoz Guzey F, Bas NS, Emel E, Alatas I, Kebudi R. Polyostotic monosystemic calvarial and spinal langerhans' cell histiocytosis treated by surgery and chemotherapy. Pediatr Neurosurg. 2003; 38:206–211.

11. Gadner H, Minkov M, Grois N, Pötschger U, Thiem E, Aricò M, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013; 121:5006–5014.

12. Haupt R, Minkov M, Astigarraga I, Schäfer E, Nanduri V, Jubran R, et al. ; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013; 60:175–184.

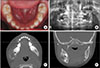
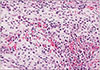

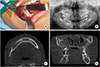




 PDF
PDF ePub
ePub Citation
Citation Print
Print



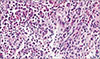

 XML Download
XML Download