Abstract
A 51-year-old male was routinely biopsied during a paraspinal muscle study. The biopsy sample was taken from the right erector spinae muscle at the fourth lumbar vertebra. The patient had no history of (diagnosed) major back trauma. The obtained sample was histologically analyzed (hematoxylin and eosin, safranin O), and complementary magnetic resonance imaging was performed. The biopsied sample contained chondroid tissue. Based on its location, the biopsy sample was appointed as chondroid metaplasia. Although chondroid metaplasia is not uncommon in humans, this is the first report of chondroid metaplasia within the paraspinal connective tissue. We propose a novel mechanism to explain the paraspinal chrondrogenic changes, related to spinal degeneration.
The paraspinal structures within the lumbar spine consist of different muscles, nerves, blood vessels, connective and adipose tissues. The extensor department of the lumbar spine can be subdivided in the three main muscles, i.e., m. Multifidus, m. Longissimus, and m. Iliocostalis [1]. These muscles are surrounded by several fascial and aponeurotic layers that separate them from the posterior abdominal wall musculature. Together these fascial and aponeurotic structures constitute the thoracolumbar fascia [2]. Fascial structures have the common property that they consist of irregular arranged collagen fibres, in contrast to aponeurotic layers or tendons, in which the collagen fibres are regular arranged [3]. Paraspinal muscles are held together by fibrous connective tissue arranged in different layers. These layers are named the endomysium, perimysium and epimysium, that can all be classified as muscle-specific fascia. All fascial tissue is composed out of a variety of different cells, that are situated within the extracellular matrix (ECM) [4]. The ECM and its progenitor cells most likely play a role in the degeneration of paraspinal muscles and their surrounding connective tissues. The primary cells identified to induce soft tissue degeneration are the mesenchymal progenitor cells, also called fibroadipogenic cells. These cells have been shown to induce fibrosis and fat infiltration within skeletal muscle tissue [5]. Fibro-adipogenic cells are suggested to contribute to the formation of extraskeletal cartilage and bone [6]. When cartilage tissue is formed in a foreign place outside the skeleton, it is diagnosed as chondroid metaplasia. Chondroid metaplasia is a benign condition, found in connective tissues in response to chronic mechanical stress [7], but can also be found in in more malignant conditions, such as breast cancer [8]. To our knowledge, chondroid metaplasia has not yet been described within paraspinal connective tissue.
The case identified in this paper pertains to a healthy participant in a study investigating structural muscle characteristics between patients with non-specific chronic low back pain and healthy controls. This study was approved by the local medical ethics committee (15.142/REVA15.14) and complies with the Declaration of Helsinki. Informed consent was given by the patient.
We report a case of chondroid metaplasia within the paraspinal tissue of a 51-year-old male, during a routine paraspinal muscle biopsy procedure [9]. The patient presented completely asymptomatic, reported no history of low back pain, and could not remember any kind of trauma related to his back. The biopsy specimen was histologically analysed, and the patient was referred to the department of radiology (Jessa Hospital, Hasselt, Belgium) for medical imaging of the spinal column.
The biopsy specimen was routinely processed and histochemically stained with hematoxylin and eosin and safranin O. The histological observations revealed the presence of a chondroid tissue. Fig. 1A and B displays the chondrocytes within their lacunae. There were no signs of increased cellularity. Due to the location and the histological aspect of the lesion, a chondrosarcoma can be excluded. Based on its histology, the sample could be misinterpreted as tissue derived from the discus intervertebralis, however this can be excluded based on biopsy site (Fig. 2).
The sample was taken at the right side of the body, at the spinal level of the fourth lumbar vertebra. The distance between the spinous process and puncture site was 40 mm, the biopsy angle was 35°, and the normal biopsy depth was 22 mm. During this biopsy the needle was not able to reach its full depth because of the resistance when penetrating the chondroid metaplasia.
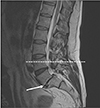
Magnetic resonance imaging (MRI) showed no deviant findings within the paraspinal musculature. At the level L4–L5 there was slight bulging of the discus superimposed with a small medial hernia, but no impression of the anterior dura. Level L5–S1 showed pronounced degenerative disc disease with pseudo bulging caused by a degree one anterolisthesis (Fig. 3). As a result of anterolisthesis and disc degeneration, caused by a bilateral isthmolysis there was bilateral foraminal stenosis. Both left and right multisegmental degenerative arthritis of the facet joints was present. Because MRI imaging could not provide a solid answer to the origin of the lesion, an ultrasonography was performed which showed a small insertional calcification on top of the iliac crest. Medical imaging could not provide us with an explanation related to our microscopic findings, but only showed severe spinal degeneration.
We describe a case of chondroid metaplasia within paraspinal connective tissue. Based on the biopsy procedure we could indicate the location of the lesion, however we could not identify its exact origin (e.g., muscles, intramuscular septum, or thoracolumbar facia). The sample did not contain muscle tissue, which makes it more likely that the we biopsied the thoracolumbar fasciae or intermuscular septum. To date there have been no reports on benign chondroid changes within paraspinal connective tissues in patients with degenerative spinal conditions. However, there have been reports on paraspinal muscle degeneration in patients with isthmic spondylolisthesis [10] and other degenerative spinal pathologies [11]. The mesenchymal derived progenitor cell might play an important role in chondroid metaplasia within the paraspinal fascia and muscle. Under normal circumstances these cells give rise to fibroblasts that produce the ECM [45]. Mesenchymal derived cells have already shown their osteogenic and chondrogenic potential [512]. It is possible that osteogenic cytokines or dysregulation of bone morphometric protein signalling within an inflammatory context could play a role in the process of transforming mesenchymal derived cells into chondroid metaplasia [6]. The role of local inflammation has already been identified through muscular changes in persons with spinal pathology, such as fibrosis and fatty degeneration [1314]. We suggest the existence of a connection between degeneration of the spine and changes within the paraspinal connective tissues. This proposition is based on the interaction between mesenchymal derived progenitor cells and local inflammation seen in spinal pathologies.
We presented the first case of chondroid metaplasia within paraspinal connective tissue, combined with severe spinal degeneration. Our microscopic findings could not be explained through medical imaging. Although in this case the chondroid metaplasia did not lead to clinical symptoms, the presence of chondroid metaplasia within the lumbar spine might be linked to spinal degeneration, trauma or strong stress and should not be neglected. To date, the clinical value of this chondroid metaplasia within the degenerative spine is not clear. Large-scale surveys using cadavers can be further needed, since chondroid metaplasia in the lower back can be discovered by chance in asymptomatic subjects.
Figures and Tables
Fig. 1
Hematoxylin and eosin staining (×40) showing the chondrocytes (black) within their lacunae indicated using white arrows. (B) Safranin O (×40) showing cartilage (red/orange) and connective tissue (green), nuclei (black); groups of chondrocytes within their lacunae are indicated using white arrows.

Acknowledgements
We would like to thank dr. Geert Souverijns from the department of radiology at the Jessa Hospital Hasselt (Belgium), for taking and interpreting the medical images.
References
1. Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980; 131:525–540.
2. Willard FH, Vleeming A, Schuenke MD, Danneels L, Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012; 221:507–536.

3. Clemente CD. Gray's anatomy of the human body. Philadelphia, PA: Lea & Febiger;1985.
4. Zullo A, Mancini FP, Schleip R, Wearing S, Yahia L, Klingler W. The interplay between fascia, skeletal muscle, nerves, adipose tissue, inflammation and mechanical stress in musculo-fascial regeneration. J Gerontol Geriatr. 2017; 65:271–283.
5. Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014; 5:68.

6. Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012; 27:1004–1017.

7. Shon W, Folpe AL. Myxochondroid metaplasia of the plantar foot: a distinctive pseudoneoplastic lesion resembling nuchal fibrocartilaginous pseudotumor and the equine digital cushion. Mod Pathol. 2013; 26:1561–1567.

8. Myint ZW, Raparla S, Kamugisha LK. Metaplastic breast cancer with chondroid differentiation. J Community Hosp Intern Med Perspect. 2015; 5:28935.

9. Agten A, Verbrugghe J, Stevens S, Boomgaert L, B OE, Timmermans A, Vandenabeele F. Feasibility, accuracy and safety of a percutaneous fine-needle biopsy technique to obtain qualitative muscle samples of the lumbar multifidus and erector spinae muscle in persons with low back pain. J Anat. 2018; 233:542–551.

10. Thakar S, Sivaraju L, Aryan S, Mohan D, Sai Kiran NA, Hegde AS. Lumbar paraspinal muscle morphometry and its correlations with demographic and radiological factors in adult isthmic spondylolisthesis: a retrospective review of 120 surgically managed cases. J Neurosurg Spine. 2016; 24:679–685.

11. Park MS, Moon SH, Kim TH, Oh J, Lee SJ, Chang HG, Shin JH. Paraspinal muscles of patients with lumbar diseases. J Neurol Surg A Cent Eur Neurosurg. 2018; 79:323–329.

12. Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ Jr, Cummins SM, Legendre NP, Yamamoto S, Kaartinen V, Hunter JW, Goldhamer DJ. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018; 9:471.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download