Abstract
Polyurethane foam (PU foam) is a new material which is being used in producing both macro-anatomical and micro-anatomical specimens. PU foam is simple to use, without need for special equipment. The present study was carried out to evaluate morphology of coronary sinus and its tributaries. During the study, we encountered few problems in carrying out injections. Coronary sinus and its tributaries were difficult to cannulate since the coronary sinus lacks a vascular stem, around which ligature can be tied before injection so that the cannula can be held in place. In contrast, in majority of the organs it is easy to inject since they possess tubular vascular stem to hold the cannula in place. A new device was developed which could be used to cannulate coronary sinus orifice to inject the casting media. The second problem we faced was saponification of adipose tissue. This made corrosion of the soft tissue difficult. Hence in this study, we describe the device we have developed to place in the coronary sinus orifice, and how saponified adipose tissue was taken care during the actual maceration step.
Casting refers to filling of anatomical or pathological spaces with extraneous material which results in the production of a three-dimensional replica of the said space [1]. In corrosion casting, the surrounding tissue of the casted hollow space is removed. The cast thus obtained represents the replica of the space of interest. Corrosion casts not only replicate the gross architecture but also the fine luminal surface morphology of the system cast [2].
The materials used for casting include gelatin, latex, silicone rubber, methyl methacrylate, epoxy resins, polyester resins [1]. Polyurethane foam (PU foam) is a new material that has been tried for this purpose, both in macrovascular and microvascular casts. Polyurethane is a polymer of organic units which are joined by urethane links. The organic units are polyethylene glycol and diisocyanate which react releasing carbon dioxide. This leads to expansion of the polymer as it solidifies. The process occurs in room temperature, and the final compound is stable. Hence, when injected the polymer expands within the cavities and reproduces the shape. PU foam is available commercially in a canister. Once injected, it was found to expand by 43% on the free surface and solidifies in a uniform manner, and hardens completely in two hours. The material is impermeable, unassailable from chemical agents, solvents and molds [3].
PU foam is a versatile material and the casts produced can be utilized in dissection courses, for display in museums as well as in scanning electron microscopy [1]. Meyer et al. (2007) [4] studied the blood vessels of the flaps (used for reconstructive surgeries) by PU foam. They compared polyurethane elastomer (PU4ii) in comparison to silicone. PU4ii was found to be better suited, as it was hard, pliable and was able to enter even smaller blood vessels. It was found to have high corrosion resistance and stability [4].
Viggiano et al. (2003) [3] described the use of PU foam to obtain vascular and bronchial endocasts of voluminous organs. PU foam was injected by compressed air, as the media is in a pressurized canister. Hence special equipment or setup was not required. But the casts produced were white in color. The casts thus obtained were lightweight, easy to handle, showed finest ramifications, and were resistant to mechanical stress [3].
De Sordi et al. (2014) [5] studied lungs, renal, intestinal of various animals and equine digital vessels. The technique was described as inexpensive, easy to carry out and did not require special apparatus. Prior formalin fixation was not required.
The technique adopted by De Sordi et al. [5] was different from that of Viggiano et al. [3]. In the latter, the foam was directly injected into the required blood vessel of the specimen of interest from the canister. Hence color could not be added. But the former researchers combined the foam and acetone in a separate glass beaker to which color could be added. The prepared resin mixture was injected with a syringe at the earliest, as it starts hardening.
In both the techniques mentioned above, 10% sodium hydroxide (NaOH) was used as the corroding agent. De Sordi et al. [5] observed saponification of adipose tissue on corrosion with NaOH and forming a thin film coat over the cast. Hence the cast required repeated rinses to remove it.
A good detergent should possess the following properties- able to lyse cells, solubilize proteins and should suitable for downstream applications. Triton-X100 used in the present study is a non-ionic surfactant. It is uncharged and has hydrophilic head groups. It is a mild surfactant and does not denature proteins. It is found to break down protein-lipid, lipid-lipid associations, but does not act on protein-protein interactions [6].
In the present paper, we describe the technique for casting the blood vessels of the heart, the problems encountered, and how they were overcome.
This study was carried out in the Research Laboratory of Department of Anatomy, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) in collaboration with the Department of Forensic Medicine and Toxicology, JIPMER from June 2015 to June 2017. The study was approved by the Post Graduate Research Monitoring Committee (vide approval No. PGRMC/ANAT/01/2015 dated 01.06.2015) and Institute Ethics Sub-committee (Human studies) (vide approval No.JIP/IEC/SC/2015/19/781 dated 17.07.2015).
The steps in preparation of corrosion cast of the blood vessels is described below.
Fresh hearts were obtained from the mortuary after informed written consent. An incision was made close to the sulcus terminalis along the right border of the heart from superior to inferior venacava and the interior of right atrium was exposed. They were thoroughly washed in running tap water till the outflow water from coronary sinus (CS) orifice becomes clear. This was achieved by tying the ascending aorta directly to the tap water source, which required approximately 20–30 minutes. The CS orifice, the Thebesian valve were evaluated. The morphological measurements were obtained at this stage.
An indigenously made cannula was used to inject the media into the CS orifice. The original endotracheal (ET) tube of different sizes (3.5, 4, and 4.5 mm) with inflatable cuff was suitable. The universal airway connector end of the ET tube was cut and removed (Fig. 1). The modified cannula was inserted into the CS orifice; the micro-cuff was inflated using an empty syringe thereby occluding the CS orifice completely so that the ET tube could not slip off and backflow of resin is prevented. This was essential, since the CS cannot be perfused directly with the regular syringe since it lacks vascular stem to hold the cannula or syringe in position.
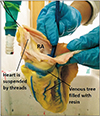
To the cut end of the cannula, the disposable syringe containing freshly prepared colored casting media was inserted. About 20 ml of media was sufficient to fill the venous tree of the heart. The media used was PU foam (Soudafoam 1K, McCoy Soudal Sealants Adhesives & Foams Pvt. Ltd., New Delhi, India) which is commercially available. It was premixed in a glass beaker with acetone (LR grade) in the ratio of 4:1 and blue color (Asian Paints universal stainer). The prepared mixture was injected slowly over 5–7 minutes till the resistance was felt. The veins getting filled with the media could be made out externally (Fig. 2). The syringe is left in place for 10 minutes, to allow resin to harden.
At this step, the bulb is deflated and the cannula was withdrawn. The CS orifice was closed for a minute with the thumb to prevent backflow. The foam material sets in 20–30 minutes. The heart was kept in the fridge (4℃) overnight for complete polymerization of the cast.
The following day, the heart was taken out and thawed. Now the arteries were also injected with PU foam media via the coronary ostia, one at a time. The aortic valve was closed using sutures, to prevent backflow of resin into the left ventricle. The details of making the media was similar as described above, except that red color (Nerolac universal stainer) was used during mixing. After injection, the heart was kept in the fridge for complete polymerization of the cast.
Next day, the heart was taken out and was immersed in 10% NaOH (1,500–1,800 ml required for one heart) for corrosion of the soft tissues. The solution was changed after 48 hours, if needed. During this process, the heart tends to float in the solution. Hence glass rods were used to keep it completely immersed.
The fat around the epicardium reacts with the NaOH to form a saponified layer of fat around the cast and the macerated tissue (Fig. 3). We tried removing it by washing and with the forceps, but it was tedious. The cast also tends to get broken during cleaning.
As the adipose tissue was responsible for saponification, Triton-X100 which is a detergent was tried to overcome this problem. The heart was immersed in 800 ml of 2% Triton-X 100 solution in a beaker of 1,000 ml capacity at 45℃ in an incubator for 24 to 48 hours for the complete dissolving of saponified tissue. The duration required depends on the size of heart, and amount of fat covering it.
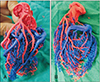
We were able to obtain excellent results in clearing the soft/adipose tissue sticking to the cast. Final cast was well preserved. It was taken out and washed thoroughly (Fig. 4).
During the procedure, there was tendency for some parts of the cast to break. They were fixed with an epoxy-based resin adhesive.
In the current study, we were able to obtain venous and arterial cast of blood vessels of the heart. The arterial cast was red colored and venous cast blue colored. The branching pattern and topographical relationship between the arterial and venous system were clearly visible in the heart cast. Even the finer vessels could be made out clearly. The main advantage of this procedure was, both arterial and venous casts could be shown in the same cast.
The technique as described above, we were able to cannulate the CS orifice with the help of modified ET tube with a syringe attached to it. It was suitable for the purpose as it had inflatable bulb near its tip, to keep it in position within the CS orifice. This technique is very easy to make and can be performed even in smaller laboratories.
Second modification followed here was the use of Triton-X 100. As described by De Sordi et al. [5], adipose tissue saponifies on maceration with NaOH. But how to overcome it was not mentioned in their protocol, except that it required repeated rinses. The problem we faced was because the heart has a variable quantity of fat on its epicardium unlike other organs in the body like lungs, kidneys, or limbs. Hence saponification was not a major problem in the protocols described by the previous researchers. As we wanted to explore the morphology of the cardiac venous tree, we encountered this problem.
Hence there was a need for detergent by which this saponified adipose tissue could be easily removed. Study by Sims and Albrecht (1993) [7] mention the use of detergents like Triton-X100, 1% 7× and 1% Terg-A-Zyme along with NaOH or potassium hydroxide in comparative study on corroding reagents.
The main advantages of this protocol are as follows: it is a low-cost, can be carried out even in small laboratories, and does not require special apparatus. The morphological details were well preserved. The casts produced are lightweight, easy to handle. Hence this technique is useful for research as well as academic purpose.
Corrosion casting with PU foam has disadvantages like-the casts are slightly brittle, due to low elasticity of PU foam and the need to procure fresh specimens. From our experience, we found it difficult to cannulate the CS, since specimen was fresh and was slippery. We need assistance to carry out the procedure. After washing and clearing the blood vessels, use of fixative could be beneficial (vascular prefixation) [8]. This area needs to be explored, so that fresh specimens can be handled with ease.
Figures and Tables
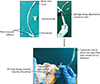 | Fig. 1Steps in the development of device. a, parts of endotracheal (ET) tube; b, syringe being attached; c, positioning of the device in the coronary sinus (CS) orifice. |
Acknowledgements
We wish to acknowledge Dr. Yogesh A. Sontakke, Associate Professor in our department for his help and suggestions during standardization of the technique. We wish to thank Dr. Dhivyalakshmi, Postgraduate in Department of Anatomy for her help in specimen preparation.
References
1. Haenssgen K, Makanya AN, Djonov V. Casting materials and their application in research and teaching. Microsc Microanal. 2014; 20:493–513.

2. Aharinejad SH. Corrosion casting: a historical review. In : Aharinejad SH, Lametschwandtner A, editors. Microvascular Corrosion Casting in Scanning Electron Microscopy. Vienna: Springer-Verlag;1992. p. 3–11.
3. Viggiano D, Sangiorgi S, Reguzzoni M, Manelli A, Marano L, Dell'Orbo C, Passiatore C. A new method to make vascular and bronchial casts of voluminous organs. Eur J Morphol. 2003; 41:161–165.
4. Meyer EP, Beer GM, Lang A, Manestar M, Krucker T, Meier S, Mihic-Probst D, Groscurth P. Polyurethane elastomer: a new material for the visualization of cadaveric blood vessels. Clin Anat. 2007; 20:448–454.

5. De Sordi N, Bombardi C, Chiocchetti R, Clavenzani P, Trerè C, Canova M, Grandis A. A new method of producing casts for anatomical studies. Anat Sci Int. 2014; 89:255–265.

6. Johnson M. Detergents: Triton X-100, Tween-20, and more [Internet]. Labome;2019. cited 2019 Jan 2. Available from: https://www.labome.com/method/Detergents-Triton-X-100-Tween-20-and-More.html.
7. Sims PA, Albrecht RM. Improved tissue corrosion of vascular casts: a quantitative filtration method used to compare tissue corrosion in various concentrations of sodium and potassium hydroxide. Scanning Microsc. 1993; 7:637–642.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download