Abstract
The anatomy and clinical significance of the sinuvertebral nerve is a topic of considerable interest among anatomists and clinicians, particularly its role in discogenic pain. It has required decades of research to appreciate its role, but not until recently could these studies be compiled to establish a more complete description of its clinical significance. The sinuvertebral nerve is a recurrent nerve that originates from the ventral ramus, re-entering the spinal canal via the intervertebral foramina to innervate multiple meningeal and non-meningeal structures. Its complex anatomy and relationship to discogenic pain have warranted great interest among clinical anatomists owing to its sympathetic contribution to the lumbar spine. Knowledge of the nerve has been used to design a variety of diagnostic and treatment procedures for chronic discogenic pain. This paper reviews the anatomy and clinical aspects of the sinuvertebral nerve.
First described by the German anatomist Hubert von Luschka in 1850, the sinuvertebral nerve has since acquired many other names including the recurrent nerve of Luschka, recurrent meningeal nerve, ramus meningeus, and meningeal branch of the spinal nerve. Luschka described the sinuvertebral nerve's derivation from the spinal nerve and its connection to the sympathetic nervous system. Furthermore, he indicated that there could be intersegmental anastomoses among its branches, but was unable to demonstrate them. His account was maintained for nearly a century until illustrations by other scientists showed that the nerve's distribution extended as far as the posterior anulus fibrosus [1234567].
It is debated whether the distribution of the sinuvertebral nerve is segmented at the level of the spinal nerve, or non-segmented with branches extending both rostrally and caudally within the spinal canal. Van Buskirk [8] described it as extending the length of the vertebral canal, with anastomosing branches above and below it. Lazorthes et al. [9] challenged those findings, describing the nerve's course as purely segmental.
Also important to many investigators was the information transmitted by the sinuvertebral nerve, and how its activity would manifest in clinical practice. Since the nerve is both somatic and autonomic, it has been investigated to determine whether it conveys discogenic pain via general visceral afferents or somatic afferents. More recently, Cavanaugh et al. [10] attempted to answer this question by stimulating the posterior surface of the L5–L6 intervertebral disc in rabbits using electrical and mechanical methods of neuronal excitation. After dissecting each grey ramus communicans, Cavanaugh et al. [10] established the general visceral afferents as the predominate pathway in lumbar discogenic pain.
Some questions regarding the sinuvertebral nerve remain unanswered. For example, some investigators have demonstrated that applying direct pressure on a diseased disc resulted in pain, while the same action on a normal disc was painless [1112]. Early in the debate, Li et al. [13] made the accurate suggestion that the diseased disc is sensitized by the growth of nerve fibers within its fissures. Shinohara [14] was the first to report nerve fibers within the deep layers of the anulus fibrosus in degenerated intervertebral discs. More recently, appreciation of the inflammatory response has helped explain those findings through various experiments demonstrating that exposure of the nucleus pulposus to the outer layers of the anulus fibrosus and neuronal tissue attracts inflammatory cells [15] and is therefore significant in the induction of hyperalgesia [16].
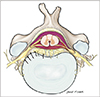
According to anatomy textbooks [1718], the sinuvertebral nerve is formed by the union of a somatic root from the ventral ramus and an autonomic root provided by the grey ramus (Fig. 1). Among the cervical levels, the grey rami give rise to the autonomic roots to form the vertebral nerve, whereas the thoracic and lumbar levels receive somatic roots from the ventral rami and autonomic contributions from grey rami communicans of the sympathetic trunk. This mixture of somatosensory and sympathetic fibers has traditionally been portrayed as a single nerve, but more accurately it comprises a series of fine filaments of which one to four larger trunks can be evident [19].
The sinuvertebral nerve arises bilaterally from the ventral ramus of each spinal nerve just distal to the dorsal root ganglia, supplying both proprioceptive and nociceptive fibers. Upon separation from the ventral ramus, it travels medially for 2–3 mm to be joined by a branch from the grey ramus communicans [20]. This branch contributes sympathetic neurons to the sinuvertebral nerve. The nerve then takes a recurrent course and re-enters the spinal canal through the intervertebral foramen, more specifically through the osteofibrous foramen formed by the deep anterior intraforaminal ligament, just caudal to the pedicle. Although it passes through the intervertebral foramen, it is unlikely to be compressed during disc herniation because it is located alongside the pedicle, cranial to the corresponding disc [20]. At the point of entrance, the composite nerve is about 0.5–1.0 mm in diameter [21].
The pathway of the sinuvertebral nerve following its passage into the spinal canal has been a topic of much debate since the 1990s. Kojima's experiments (1990) [56] on rats concluded that the nerve divides into superficial and deep networks around the posterior longitudinal ligament. The deep network was said to be segmental, supplying sensation to the posterior anulus, while the superficial network was determined to be non-segmental with both ascending and descending branches passing through several levels. Kojima's experiments showed that the superficial network comprises predominately sympathetic nerves while the deep network is primarily somatic. However, Nakamura et al. [22] showed that lumbar sympathectomy resulted in 90% loss of sensory innervation to the posterior anulus fibrosus, indicating that the nerve was largely sympathetic.
Findings based on work by Imai et al. [23] using immunoreactive staining for tyrosine vasoactive intestinal polypeptide and substance P [24] demonstrated postganglionic sympathetic fibers in the posterior longitudinal ligament [25]. Additionally, work by Konttinen et al. [26] and Coppes et al. [24] using calcitonin gene-related peptide and substance P, showed the presence of nociceptive fibers in both superficial and deep divisions of the sinuvertebral nerve as it courses along the posterior longitudinal ligament. Moreover, tyrosine hydroxylase immunoreactive staining responded only to the superficial network, verifying Kojima's [56] and Nakamura's [22] previous findings that the superficial network was primarily sympathetic.
The next innovation in the investigation of the sinuvertebral nerve involved the use of retrograde transport markers cholera toxin B and horseradish peroxidase crystals by Morinaga et al. [27]. In Morinaga et al.'s experiment, the two markers were injected into the anterior L5–L6 intervertebral discs of rats followed by histological examination of the dorsal root ganglia. Surprisingly, labeled neurons appeared to be restricted to the L1–L2 level [27]. On this basis it was hypothesized that the nociceptive fibers passed through the sympathetic trunk from L5–L6 to L1–L2, an inference later supported by Sekiguchi et al.'s demonstration [28] of increased pain threshold following sympathectomy. However, this did not help to explain the sinuvertebral nerve's role in discogenic pain, as it was widely accepted that sympathetic nerves directly from the sympathetic trunk only innervated the anterior anulus fibrosis. A similar experiment by Cavanaugh et al. [10] on the posterior aspect of the anulus verified a clear nociceptive ascending track along sympathetic afferents from lower lumbar levels.
The sinuvertebral nerve also innervates a number of additional structures, one being the anterior portion of the dura mater within the spinal canal. Each sinuvertebral nerve sends a long descending meningeal branch that extends two segments caudally and a shorter ascending branch that traverses as far as one rostral segment. The plexus formed by the anastomoses of these branches covers the ventral surface of the dura mater and extends to the lateral aspects, but never reaches the dorsal surface, which remains devoid of nerve supply. The three most rostral sinuvertebral nerves ascend through the foramen magnum, at which point they innervate the dura mater covering the clivus within the posterior cranial fossa.
The sympathetic fibers carried in the sinuvertebral nerve are thought to innervate much of the surrounding vasculature, including the vessels that supply blood to the outer anulus, end plates, vertebral bodies, and marrow. It has been speculated that these fibers are primarily involved in vasomotor regulation, although some have been found to travel distal to the blood vessel, suggesting an additional undetermined function [29].
Finally, sinuvertebral nerves have also been found to terminate in the periosteum of the vertebrae as well as the ligaments of the zygapophyseal and median atlanto-axial joints. The sinuvertebral nerve does not supply sensation to the facet joint, another common source of chronic back pain said to be mediated primarily by the medial branch of the posterior ramus [30]. Despite the different etiology, facet joint and discogenic pain cannot be distinguished clinically without specific diagnostic procedures [31]. The sinuvertebral nerve's sensory supply to the median atlanto-axial joint has been questioned recently as a potential cause of chronic headaches [3233]. While osteoarthritis of the median atlanto-axial joint can be detected, it has not yet been associated with headache. Additionally, no techniques have yet been developed to distinguish between symptomatic and asymptomatic osteoarthritic changes to the median atlanto-axial joint.
Low back pain is a common disability among the general population. It is said to cost about $24 billion annually in the United States and is the leading cause of compensated injury in the workplace. An estimated 84% of the general population is expected to experience low back pain at least once in their lives. There are multiple potential sources of chronic low back pain, most often divided into facet-mediated, fracture, and discogenic pain. The sinuvertebral nerve, associated with the discogenic type (pain from an injured vertebral disc), accounts for approximately 26%–39% of patients with low back pain [3435]. This pain travels through the rami communicantes down to L2 where it joins the sympathetic ganglion and then travels to the skin at lower levels.
Although the sinuvertebral nerve fibers are said to terminate in the outer anulus, it is now widely accepted that this end point is not permanent. Studies of healthy patients normally show neural penetration of the anulus at about 3 mm [36], corresponding to the three outer lamellae [37]. However, degenerative discs have shown penetration of nerve fibers as far as the inner one third in one study [38], and into the nucleus pulposus in another [39]. Earlier studies focused on the findings of the herniated nucleus pulposus and the sinuvertebral nerve fibers. Various studies have demonstrated that upon contact with the nucleus pulposus, nerve fibers showed reduced spinal nerve root conduction velocities, induced nerve degeneration[40], increased nerve discharge [40], increased intraneural capillary permeability [16], and attraction of inflammatory cells [41].
Additional research has detected nerve fibers extending through tears within the anulus of degenerative discs via vascularized granulation tissue [2639], which are thought to be the pathoanatomical reason for low back pain in patients with degenerative disc disease. Moreover, nerve fibers within the endplates of degenerative discs are denser than the discs of healthy persons [42]. Investigators have proposed the release of neurogenic factors from the neurotrophin family within the degenerative disc. Purmessur et al. [43] supported this notion by showing an increase in neurotrophins in patients suffering from discogenic pain. Furthermore, Kokubo et al. [44] found that increased expression of nerve growth factor, a neurotrophin, was associated with hyperinnervation of intervertebral discs. It has also been speculated that brain-derived neurotrophic factor is implicated in the hypersensitization experienced in discogenic pain [4345]. Occasionally, leakage of inflammatory cytokines produced in annular tears into the epidural space can injure adjacent nerves, leading to radicular pain down the lower limb in the absence of disc herniation [46].
However, discogenic pain is not limited to the lower back. Overactivation of the C1–C3 sinuvertebral nerves is now considered the cause of most cervicogenic headaches. Cervicogenic headaches, once called occipital neuralgia, were first described by Sjaastad et al. [47] as recurrent, long lasting, severe unilateral headaches arising from the neck. Typically, the C2–C3 and C3–C4 intervertebral discs are implicated in cervicogenic headaches, no disc below C4–C5 resulting in referred pain to the head [48]. Nonetheless, research on headaches associated with sinuvertebral nerve stimulation is currently weak and more investigation is needed before a definitive relationship can be established.
Several diagnostic and treatment procedures have been developed to manage the pathological effects of the sinuvertebral nerve. Provocative discography is the gold standard for back pain related to intervertebral disc pathology. The procedure involves the injection of contrast medium into the disc with concomitant assessment of the patient's pain response. If a particular disc is painful, then stressing it should reproduce the patient's usual pain. If the disc is not the source of a patient's pain, then stressing it should either not be painful or produce pain to which the patient is not accustomed. Thus, patients who suffer from severe back or neck pain of unknown etiology are often recommended for provocation discography. If the intervertebral disc is implicated, the excessive pain felt during injection is transmitted via the sinuvertebral nerve. Discography has proved significantly more effective for diagnosing degenerative disc disease than magnetic resonance imaging [49]. Through various methods of blocking the sinuvertebral nerve, clinicians have been able to reduce chronic pain in some patients with degenerative disc disease. Analgesic discography is one such technique. An anesthetic (4% xylocaine or 0.75% bupivacaine) is injected into the disc to relieve pain by blocking sinuvertebral nerve conduction [50]. Another method is intradiscal electrothermal annuloplasty (IDET), where a catheter is inserted into the affected disc and a wire heated to 90℃ seals ruptures in the anulus while burning nerve fibers. In a 1-year pilot study by Derby et al. [51], 62.5% of patients had a favorable outcome following IDET. Another technique is transforaminal epiduroscopic laser ablation (TELA) of the sinuvertebral nerve. Patients with Pfirrmann disc degeneration grade IV were treated with TELA (targeting the sinuvertebral nerve), and their outcome was measured using the visual analog scale (VAS), Macnab criteria, and the Oswestry Disability Index (ODI). The results showed a significant decrease in VAS and ODI, and a 96.1% good to excellent outcome according to Macnab criteria [52].
Finally, there has been some success with radiofrequency neurotomy/ablation of the nerve. This procedure involves using energy within a certain radiofrequency range to cause selective necrosis of specific nerves to relieve pain. It entails risks such as infection, hematoma formation, burns, and neural injury including cutaneous hypoesthesia, but it is simple and can be performed as an outpatient procedure with patient discharge just hours afterward.
Cervicogenic headaches, like low back pain, can be diagnosed by provocative discography [53]. Radiofrequency neurotomy of the C3–C4 nerve root outer layers, including the sympathetic nerve fibers of the sympathetic trunk, has been reported to have considerable success in reducing the intensity and frequency of cervicogenic pain [54].
Recent studies have shown that the sinuvertebral nerve can be traced as far as the outer three layers of the lamella in healthy patients, but can go as far as the nucleus pulposus in degenerative discs. The nerve has also been implicated in discogenic pain. Yet only recently has its role been extensively studied, providing opportunities for the development of newer diagnostic and treatment techniques to combat the debilitating consequences of its pathology. Provocative discography is still the gold standard for diagnosing discogenic pain. While these procedures have proved advantageous in discogenic pain management, much more work needs to be done to improve their accuracy and efficacy. The sinuvertebral nerve has also been linked to cervicogenic headaches, but more research is needed to clarify its involvement in this condition.
Figures and Tables
References
2. Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007; 89:1135–1139.

3. Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981; 132:39–56.
4. Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine (Phila Pa 1976). 1988; 13:2–8.

5. Kojima Y, Maeda T, Arai R, Shichikawa K. Nerve supply to the posterior longitudinal ligament and the intervertebral disc of the rat vertebral column as studied by acetylcholinesterase histochemistry. II. Regional differences in the distribution of the nerve fibres and their origins. J Anat. 1990; 169:247–255.
6. Kojima Y, Maeda T, Arai R, Shichikawa K. Nerve supply to the posterior longitudinal ligament and the intervertebral disc of the rat vertebral column as studied by acetylcholinesterase histochemistry. I. Distribution in the lumbar region. J Anat. 1990; 169:237–246.
7. Groen GJ, Baljet B, Drukker J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien). 1988; 92:39–46.

8. Van Buskirk C. Nerves in the vertebral canal: their relation to the sympathetic innervation of the upper extremities. Arch Surg. 1941; 43:427–432.
9. Lazorthes G, Poulhes J, Espagno J. Etude sur les nerfs sinuvertebraux lombaires. Le nerf de roofe existe-t-il? C R Assoc Anat. 1947; 34:317–320.
10. Cavanaugh JM, Ozaktay AC, Yamashita T, Avramov A, Getchell TV, King AI. Mechanisms of low back pain: a neurophysiologic and neuroanatomic study. Clin Orthop Relat Res. 1997; (335):166–180.

11. Lindblom K. Technique and results of diagnostic disc puncture and injection (discography) in the lumbar region. Acta Orthop Scand. 1951; 20:315–326.

12. Perey O. Contrast medium examination of the intervertebral discs of the lower lumbar spine. Acta Orthop Scand. 1951; 20:327–334.

13. Li YR, Hu X, Yang BZ. Studies on structural changes of collagen in silicosis. Biomed Environ Sci. 1994; 7:302–306.
14. Shinohara H. Lumbar disc lesion, with special reference to the histological significance of nerve endings of the lumbar discs. Nihon Seikeigeka Gakkai Zasshi. 1970; 44:553–570.
15. Olmarker K, Blomquist J, Strömberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine (Phila Pa 1976). 1995; 20:665–669.

16. Byrod G, Otani K, Brisby H, Rydevik B, Olmarker K. Methylprednisolone reduces the early vascular permeability increase in spinal nerve roots induced by epidural nucleus pulposus application. J Orthop Res. 2000; 18:983–987.
17. Standring S. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. New York: Elsevier;2016. p. 734–767.
18. Moore KL, Agur AM, Dalley AF. Essestial clinical anatomy. 5th ed. Philadelphia, PA: Wolters Kluwer;2015. p. 285.
19. Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990; 188:282–296.

20. Edgar MA, Ghadially JA. Innervation of the lumbar spine. Clin Orthop Relat Res. 1976; (115):35–41.

21. Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1949; 19:211–221.

22. Nakamura S, Takahashi K, Takahashi Y, Morinaga T, Shimada Y, Moriya H. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 1996; 21:917–924.

23. Imai S, Hukuda S, Maeda T. Dually innervating nociceptive networks in the rat lumbar posterior longitudinal ligaments. Spine (Phila Pa 1976). 1995; 20:2086–2092.

24. Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997; 22:2342–2349.

25. Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine (Phila Pa 1976). 1993; 18:268–273.

26. Konttinen YT, Grönblad M, Antti-Poika I, Seitsalo S, Santavirta S, Hukkanen M, Polak JM. Neuroimmunohistochemical analysis of peridiscal nociceptive neural elements. Spine (Phila Pa 1976). 1990; 15:383–386.

27. Morinaga T, Takahashi K, Yamagata M, Chiba T, Tanaka K, Takahashi Y, Nakamura S, Suseki K, Moriya H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine (Phila Pa 1976). 1996; 21:1848–1851.

28. Sekiguchi Y, Konnai Y, Kikuchi S, Sugiura Y. An anatomic study of neuropeptide immunoreactivities in thelumbar dura mater after lumbar sympathectomy. Spine (Phila Pa 1976). 1996; 21:925–930.
29. Suseki K, Takahashi Y, Takahashi K, Chiba T, Yamagata M, Moriya H. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes: a possible pathway for discogenic low back pain. J Bone Joint Surg Br. 1998; 80:737–742.
31. Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003; 14:455–472.

32. Harata S, Tohno S, Kawagishi T. Osteoarthritis of the alantoaxial joint. Int Orthop. 1981; 5:277–282.
33. Zapletal J, Hekster RE, Straver JS, Wilmink JT. Atlanto-odontoid osteoarthritis: appearance and prevalence at computed tomography. Spine (Phila Pa 1976). 1995; 20:49–53.
34. Manchikanti L, Singh V, Pampati V, Damron KS, Barnhill RC, Beyer C, Cash KA. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001; 4:308–316.
35. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995; 20:1878–1883.

36. Ashton IK, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs: binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994; 65:635–639.

37. Palmgren T, Gronblad M, Virri J, Kääpä E, Karaharju E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976). 1999; 24:2075–2079.

38. McCarthy PW, Carruthers B, Martin D, Petts P. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine (Phila Pa 1976). 1991; 16:653–655.

39. Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005; 87:62–67.

40. Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine (Phila Pa 1976). 2001; 26:940–945.

41. Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: a review. Spine Surg Relat Res. 2018; 2:11–17.

42. Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003; 28:2570–2576.

43. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in thenondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008; 10:R99.
44. Kokubo Y, Uchida K, Kobayashi S, Yayama T, Sato R, Nakajima H, Takamura T, Mwaka E, Orwotho N, Bangirana A, Baba H. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J Neurosurg Spine. 2008; 9:285–295.
45. Bennett GJ. Are the complex regional pain syndromes due to neurogenic inflammation? Neurology. 2001; 57:2161–2162.

47. Sjaastad O, Saunte C, Hovdahl H, Breivik H, Grønbaek E. “Cervicogenic” headache: an hypothesis. Cephalalgia. 1983; 3:249–256.

48. Slipman CW, Lipetz JS, Jackson HB, Plastaras CT, Vresilovic EJ. Outcomes of therapeutic selective nerve root blocks for whiplash induced cervical radicular pain. Pain Physician. 2001; 4:167–174.
49. Choi SH, Adsul N, Kim HS, Jang JS, Jang IT, Oh SH. Magnetic resonance imaging undetectable epiduroscopic hotspot in chronic diskogenic back pain: does sinuvertebral neuropathy actually exist? World Neurosurg. 2018; 110:354–358.
50. Schliessbach J, Siegenthaler A, Heini P, Bogduk N, Curatolo M. Blockade of the sinuvertebral nerve for the diagnosis of lumbar diskogenic pain: an exploratory study. Anesth Analg. 2010; 111:204–206.
51. Derby R, Eek B, Chen Y, O'Neill C, Ryan D. Intradiscal electrothermal annuloplasty (IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000; 3:82–88.

52. Kim HS, Paudel B, Chung SK, Jang JS, Oh SH, Jang IT. Transforaminal epiduroscopic laser ablation of sinuvertebral nerve in patients with chronic diskogenic back pain: technical note and preliminary result. J Neurol Surg A Cent Eur Neurosurg. 2017; 78:529–534.

54. Blume HG. Cervicogenic headaches: radiofrequency neurotomy and the cervical disc and fusion. Clin Exp Rheumatol. 2000; 18:2 Suppl 19. S53–S58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download