Abstract
Ossification of the mamillo-accessory ligament (MAL) is a misunderstood phenomenon; however, many have posited that it can result in nerve entrapment of the medial branch of the dorsal ramus causing zygapophyseal joint related low back pain. The MAL has been studied anatomically by few, yet the data indicate possible associations between ossification of this ligament and spondylosis. It has been proposed that mechanical stress upon the lumbar spine may also lead to progressive ossification of the MAL into a bony foramen.
The mammillary (MP) and accessory processes (AP) are bony projections of the lumbar vertebrae; the MP is a round bony protrusion found at the posterior borders of the superior articulating facets and the AP is a relatively smaller and rough knob located along the posteroinferior aspect of the base of each transverse process (Fig. 1). The tips of the ipsilateral MP and AP are bridged via a fibrous connective band called the mamillo-accessory ligament (MAL), though actually considered a “false” ligament as it joins aspects of the same bone [12345]. The MAL has no notable biomechanical significance; however, its primary purpose is to provide a collagenous slip for the medial branch of the dorsal ramus to course beneath [1]. Like other “false” ligaments, the MAL may undergo ossification (Fig. 1) of varying degrees: partial, where spurs of bone from the AP reach posteromedially to the ipsilateral MP and orthogonal to the path of the medial branch of the dorsal ramus; complete, wherein a osteogenic bridge replaces a significant portion of the MAL [3].
Ossification of the MAL may be a potential origin of chronic low back pain arising from the zygapophyseal (facet) joints, and may cause difficulties in orientation and administration of anesthetics for facet nerve blockade [67]. Herein, we provide a review of the extant literature regarding patterns and degrees of ossification of the MAL, including its anatomy, morphological and functional relationships with adjacent neural and musculoligamentous structures, and insight into its clinical importance.
An electronic database (MEDLINE) and public search domain (Google Scholar) were searched with access dates between July 30, 2018 and August 2, 2018. The key words used are listed as follows: MAL ossification, facet joint pain, facet nerve blockade techniques, lumbar spine anatomy, lumbar spine biomechanics, and lumbar spine loading. Anatomical studies of the MAL, as well as general studies mentioning ossification of the MAL were searched and reviewed. Studies in journals that did not have accessible English versions were excluded from this review. Techniques of medial branch blockade and denervation of the medial branch of the dorsal ramus were searched and reviewed. Literature including the phrases “mamillo-accessory ligament ossification” and “low back pain” were concentrated on, but there were no significant exclusion criteria.
A total of 33 references were reviewed fully or by abstract when full text was unavailable. Of these, four were anatomical studies directly pertaining to the ossification of the MAL, the most recent study published in 2014. One biomechanical study was obtained, and four book chapters detailing lumbar vertebral anatomy and clinical techniques regarding lumbar facet nerve blocks were also reviewed. To our knowledge there is no literature discussing the precise nature of ossification of the MAL, however spondylosis, and mechanical stress are both plausible origins.
The MAL is a fibrous cord (1–2 mm thickness) that interfaces with the superior aspect of the tip of the AP and the ipsilateral MP of each lumbar vertebra, and are more noticeably developed at the L1–L4 levels than L5 [3]. There are three distinct anatomical surfaces of the MAL that interface with the neuromuscular system: the dorsal surface acts as a connective substrate for the lateral fascicles of the multifidus; the ventral surface covers the medial branch of the dorsal ramus of the superior lumbar vertebral level; the lateral surface serves as a substrate for the medial fascicles of the longissimus thoracis inserting at the tips of the AP, and proceed through the MAL to attach onto the MP.
The term “mamillo-accessory ligament” was coined by Bogduk [3]. The degrees of ossification were considered: “nil,” no observable spicules; “partial,” featuring observable bony spicules; and “complete,” wherein a complete bar of bone at varying thickness was observed. A mamillo-accessory notch (MAN) has also been described as a consistent anatomical feature of the lumbar vertebrae, whereby a wide indentation formed by the MP and AP provide a saddle for the path of the medial branch of the dorsal ramus [8]. The medial branches of dorsal rami of L1–L4 cross the superior aspect of their transverse processes and pierce the intertransverse ligament at the base of the transverse process. The nerve then courses through the MAN bounded by the ventral surface of the MAL, and continues posteroinferiorly along the vertebral lamina between the superior articulating facet and transverse process, conveying sensory branches to the superior and inferior ipsilateral zygapophyseal joint capsules, intertransversarium mediales, interspinous and and multifudus muscles [39]. The MAL is a consistent structure anatomically, but its own morphological characteristics as well as the geometry of the MAN tend to change with increasing ossification. Cases of complete ossification lead to the formation of a mamillo-accessory foramen (MAF) (Fig. 2) that interferes with the nerve from being accessed surgically at that landmark [8].
Though included in some textbooks [11011], the MAL has received little attention in the literature aside from reports of its relationship with the medial branch of the dorsal ramus and possible role in facet joint pain [2361213]. There have been few morphological studies that attempt to establish connections between the configurations and ossification patterns of the MAL and lumbar nerve posterior rami entrapment causing zygapophyseal joint pain; however, the proposed association requires further investigation and discussion to establish a clinico-pathological connection [381314].
The reported incidences from these anatomical studies follow an interesting trend in the gradual ossification of the MAN to an MAF, wherein the occurrence of open notches (nil or no notch) decreased caudally and the occurrence of partial notch and MAF formation increased caudally. Both Nighsia Medical College [14] (n=100) and Bogduk [3] (n=293) demonstrate increasing ossification and formation of the MAF more caudally (L3, 7/200; L4, 21/200; L5, 55/200; and L3, 3/116; L4, 3/168; L5, 17/122). From Maigne et al. (n=193) [13], the occurrence of the MAF also increased more caudally (L3, 8; L4, 21; L5, 77), occurring most frequently at L5 on the left side. The most recent anatomical study performed by Mahato [8] demonstrated a pattern in line with previous anatomical studies describing the occurrence of MAF ossification more caudally (L3, 6/72; L4, 8/92; L5, 10/112).
Various biomechanical studies have demonstrated that facet joints are able to transmit vertical compressive forces along the spinal column, and given the wealth of literature describing the participation of the lumbar spine and lumbosacral osseous and ligamentous components in reception and stabilization against these forces, it is likely that loads incidentally transmitted down the facets and into the adjacent ligamentous structures like the MAL may lead to ossification [1516]. It had been proposed by Bogduk [3] that spondylosis may be a cause of ossification of the MAL, but there were no younger lumbar spine specimens free from spondylosis or elderly control groups implemented to provide a statistically significant comparison.
Interestingly, we found one single anatomical study by Maigne et al. (1991) [13] that included lumbar vertebrae from infants and young adults. The complete MAF or enlargement of the notch were not seen in the young adults nor the infants studied, lending substantial credence to our assertion that the ossification of this ligament is due to stress or degeneration [13]. The causes of low back pain are multifactorial, but a relationship can be proposed between compressive forces along the proximal facet joints, demonstrated to be a receptacle for vertical compressive and shear, and the ossification of the MAL and further spondylosis of neighboring vertebral components [17]. We propose a mechanism for the ossification of this ligament based on the general trends in the literature such as (1) repeated lumbar spinal shear from occupational physical loading, (2) general age-related spondylosis, and (3) the L4–L5 segments being the most ossified due to their receiving substantial compressive forces during load bearing activities [1516181920]. Further anatomical studies would need to be performed focusing exclusively on the lumbar spines and MAL of children and adolescents to elucidate these suggestions. The relationship between occupational physical loading and ossification of the MAL may also be determined by cadaveric studies examining the MAL of an elderly population with an occupational history of high physical exertion with complaints of chronic low back pain, compared to the MAL of an elderly control group with occupational history of low to no physical exertion and a history of insignificant to low grade back pain.
Osteoarthiritic changes were observed in Maigne's study only in the lumbar segments with significant notching, and or complete formation of the MAF, again providing evidence for a degenerative origin [13]. Furthermore, L3–L5 are the intervertebral joints that exhibit free flexion and extension, capable of undertaking shear due to the inherent curvature of the lumbar spine. The L4 and L5 vertebral segments also undergo the greatest amount of motion amongst the lumbar vertebrae, specifically between the facets and the vertebral bodies, allowing higher magnitudes of tension and stress distributed along these structures [1921]. The mechanics of the facet joints are not fully agreed upon, as some conclude that they are principally stabilizing joins; however, there are suggestions that support their role as weight-bearing, load dissipating, shear resisting structures [19]. The MAL acts as an interface not only for the AP and MP but for the fasciae of the multifidus, intertransversarium, and interspinous musculature, thus it would be reasonable to consider the various forces being transmitted into the ligament and further stressing to contribute to its ossification. Paravertebral ossification processes, such as a diffuse idiopathic skeletal hyperostosis, may result in the ossification processes of ligaments and could be connected to the ossification of the MAL, as a general response to biomechanical stress to the facet joints, especially in specimen where there are enthesophytes and osteophytes about the tissue neighboring the MAL [22].
The nerve itself is not visible during anesthetic blockade, however the osseous substructures of the lumbar vertebra(e) intended to be blocked are visible through radiography and are used to consistently provide injections without complications. Currently, fluoroscopy as well as ultrasound guided nerve blockade are provided with a greater success rate [423]. As discussed in the anatomy subsection, the MAL provides an osseofibrous tunnel by which the medial branch of the dorsal ramus courses through; thus, the MAL is a useful landmark for percutaneous techniques to provide stimulation, pain block, and or ablation of the branch as opposed to blind radiofrequency ablation [12]. Though the mechanism is not well understood, ossification of this ligament does not present as a complication, as the archetypal target for medial branch blockade is the space between the posterior aspect of the superior articulating facet and the MAN [24]. If there is complete ossification of the MAL, the injection may be administered at any other point of the nerve, whether superior or inferior to the MAF [25]. Though ossification may not pose as a threat in execution of clinical procedures, there still is question of whether this phenomenon occurs solitarily or is related to, and may also cause low back pain in response to mechanical stress and spondylosis.
As seen in the case of a MAF, knowledge of the anatomical variations in the region of the lumbar spine can have potential clinical implications such as diagnosing medically recalcitrant back pain with no obvious structural disease [26].
Connections between ossification of the MAL and ageing have been demonstrated; however, further investigation is needed to conclude if there is a mechanical stress related and a precise degenerative mechanism that causes this phenomenon. We conclude that though there is not compelling evidence for compression and or entrapment of the medial branch of the dorsal ramus via ossification of the MAL, its presence still may interfere with clinical procedures such as medial nerve branch blockade and denervation, thus we believe it is paramount that the clinician be aware of this phenomenon as well as be able to appreciate the anatomy of the MAL and neighboring structures for the best chances of pain relief in patients.
Figures and Tables
Fig. 1
Posterior view of the lumbar spine focused on L2 vertebra. Note on the left side of the figure, the normal positions of the mamillary (orange arrow) and accessory (white arrow) tubercles. On the right side of the figure, an ossified mamillo-accessory ligament is seen (yellow arrow) resulting in a bony foramen (i.e., the mamillo-accessory foramen) for the potential transit of the medial branch of the dorsal ramus.
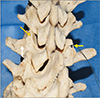
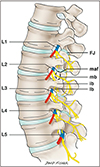
Fig. 2
Schematic drawing of the dorsal rami (yellow) of the lumbar region and their relationships to the vertebrae. Note the spinal nerve (blue) as it leaves the intervertebral foramen and the medial (mb), lateral (lb), and intermediate (ib) branches of the dorsal rami. Also note the medial branches, which are susceptible to compression by an ossified mamillo-accessory ligament traveling to the facet joints (FJ). The space for the mamillo-accessory foramen is designated by the dashed circle (maf ) with the enclosed medial branch of the dorsal ramus.
References
1. Bogduk N. Clinical and radiological anatomy of the lumbar spine. New York: Elsevier Health Sciences;2012.
2. Vetter M, Oskouian RJ, Tubbs RS. “False” ligaments: a review of anatomy, potential function, and pathology. Cureus. 2017; 9:e1853.

3. Bogduk N. The lumbar mamillo: accessory ligament. Its anatomical and neurosurgical significance. Spine (Phila Pa 1976). 1981; 6:162–167.
4. Provenzano DA. Lumbar facet nerve blocks: ultrasound. In : Narouze SN, editor. Multimodality Imaging Guidance in Interventional Pain Management. Oxford: Oxford University Press;2016. p. 254–264.
5. Francois RJ, Bywaters EG, Aufdermaur M. Illustrated glossary for spinal anatomy. With explanations and a French and German translation. Rheumatol Int. 1985; 5:241–245.
6. Odonkor CA, Shin BC, Cohen SP. The effect and role of steroids in facet joint radiofrequency denervation: a narrative review. Curr Phys Med Rehabil Rep. 2017; 5:180–185.

7. Moriggl B. Spine sonoanatomy for pain physicians. In : Narouze SN, editor. Atlas of Ultrasound-Guided Procedures in Interventional Pain Management. New York, NY: Springer;2018. p. 59–81.
8. Mahato NK. Mamillo-accessory notch and foramen: distribution patterns and correlation with superior lumbar facet structure. Morphologie. 2014; 98:176–181.

10. Standring S. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. New York: Elsevier;2016.
11. Singh H, Chang Chien G, Bolash R. Anatomy of the spine. In : Pope JE, Deer TR, editors. Treatment of Chronic Pain Conditions. New York, NY: Springer;2017. p. 11–20.
12. Gore S. Lumbar facet denervation for degenerative symptomatic functional spinal unit: overview. J Orthop Allied Sci. 2018; 6:8–12.

13. Maigne JY, Maigne R, Guerin-Surville H. The lumbar mamillo-accessory foramen: a study of 203 lumbosacral spines. Surg Radiol Anat. 1991; 13:29–32.

14. Anatomical observations on lumbar nerve posterior rami. Chin Med J (Engl). 1978; 4:492–496.
15. Panjabi MM, Goel VK, Takata K. Physiologic strains in the lumbar spinal ligaments: an in vitro biomechanical study 1981 Volvo Award in Biomechanics. Spine (Phila Pa 1976). 1982; 7:192–203.
16. Videman T, Nurminen M, Troup JD. 1990 Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine (Phila Pa 1976). 1990; 15:728–740.

17. Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine (Phila Pa 1976). 1984; 9:557–565.

18. Schultz A, Andersson G, Ortengren R, Haderspeck K, Nachemson A. Loads on the lumbar spine: validation of a biomechanical analysis by measurements of intradiscal pressures and myoelectric signals. J Bone Joint Surg Am. 1982; 64:713–720.

19. Bellew JW. Lumbar facets: an anatomic framework for low back pain. J Man Manip Ther. 1996; 4:149–156.

20. Jay G. Neuropathic low back pain: practical guide to chronic pain syndromes. Boca Raton, FL: CRC Press;2016.
21. Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. J Bone Joint Surg Am. 2006; 88:Suppl 2. 63–67.

22. Ehara S, Shimamura T, Nakamura R, Yamazaki K. Paravertebral ligamentous ossification: DISH, OPLL and OLF. Eur J Radiol. 1998; 27:196–205.

23. Shim JK, Moon JC, Yoon KB, Kim WO, Yoon DM. Ultrasound-guided lumbar medial-branch block: a clinical study with fluoroscopy control. Reg Anesth Pain Med. 2006; 31:451–454.

24. DePalma M. iSpine: evidence-based interventional spine care. New York: Demos Medical Publishing;2011.
25. Dreyfuss P, Schwarzer AC, Lau P, Bogduk N. Specificity of lumbar medial branch and L5 dorsal ramus blocks: a computed tomography study. Spine (Phila Pa 1976). 1997; 22:895–902.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download