Dear Editor,
Diphtheria was once a major cause of pediatric mortality, but its incidence has been declining since the introduction of toxoid vaccines. A recent resurgence due to various factors has been reported worldwide, with nearly 60% of the cases occurring in India.1 The most-well-known effects of the diphtheria toxin are cardiomyopathy and polyneuritis.2 Diphtheric encephalitis (DE) is a rare complication whose clinicoradiological features have seldom been reported. We describe the clinical and neuroimaging features in one such case of DE that manifested with a secondary movement disorder (SMD).
A 14-year-old boy with a history of incomplete vaccination presented with a 2-week history of high-grade fever, sore throat, and painful swallowing. His clinical course deteriorated with the development of stridor, increased drowsiness, and involuntary limb movements.
A clinical examination revealed a febrile child with enlarged congested tonsils covered by a whitish pseudomembrane. He responded poorly to stimulation and required emergent intubation. A neurological examination revealed rigidity and dystonic posturing that was most prominent in the upper limbs, along with finger tremors. Bulbar palsy could not be examined sufficiently. He also had clinical signs of mild myocarditis with persistent tachycardia and global hypokinesia with a normal cardiac size in echocardiography. No electrocardiographic changes or cardiac enzyme elevations were present. Diphtheria antitoxin was administered after throat swab cultures confirmed the presence of Corynebacterium diphtheriae and ruled out other organisms such as streptococcus.
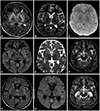
Noncontrast MRI was performed in the third week of his illness for the prognostication of encephalitis, which revealed vasogenic edema in the corpus striatum and substantia nigra along with subtle hyperintensities in the thalami and insulae (Fig. 1). Cerebrospinal fluid (CSF) studies showed elevated cell counts (110 cells/mm2, all lymphocytes) and protein (766 mg/dL), with normal glucose levels and sterile cultures.
The child required ventilation support for a further 2 weeks, during which his level of consciousness gradually improved, but more-intense extrapyramidal symptoms persisted (Supplementary Video 1 in the online-only Data Supplement). He was started on levodopa and trihexyphenidyl, which produced a significant symptomatic improvement, and he was fully ambulant with minor intellectual impairment at a 2-month follow-up (Supplementary Video 1 in the online-only Data Supplement).
Infections account for up to a fifth of SMDs, with the most-common subtype being pure dystonia or dystonia combined with other disorders.3 A wide variety of underlying bacterial and viral pathogens have been reported.3 These usually occur in the pediatric age group and manifest within 1 month of a febrile illness, although the latency period can vary widely.3 Neuroimaging commonly demonstrates involvement of the basal ganglia and thalamus, with variable involvement of the midbrain, cortex, and white matter.4
An association between DE with SMD has not been reported previously. DE itself is a rare entity, with hitherto only seven cases being reported, of which six were in the preimaging era.5678 Radiological findings were included in only one case report, which was of a 6-year-old Malaysian child with encephalitis and cerebellar signs. MRI in that case revealed features of cerebellitis with involvement of the insular and cingulate gyri.8 Preimaging era necropsy studies of DE have documented degenerative changes with or without hemorrhage variably involving the cerebrum, brainstem, spinal cord, and striatum.79
While the CSF showed an inflammatory picture in the present case, the radiological pattern of involvement was more symmetric than expected for a direct bacterial invasion. Further, the varying patterns of involvement reported in DE are consistent with the hypothesis that cross-reactivity with the toxin is the most likely pathogenesis.10 Management of infection induced SMDs in the acute phase requires the early administration of antibiotics and antitoxins, the variable administration of steroids, and symptomatic management tailored to the SMD subtype.11 The response is often satisfactory, as in the present case.
Our case highlights a novel manifestation of diphtheria and underscores the common mechanisms of autoimmunity that play a role in infection-induced SMDs.
Figures and Tables
Fig. 1
Findings of MRI performed during week 3 of the illness. FLAIR (A) and T2-weighted (B) images show bilateral striatal edema and posterior thalamic edema (arrows) without abnormal mineralization on susceptibility-weighted images (C) or diffusion restriction on diffusion-weighted images (D, E). More inferior sections at the level of the midbrain reveal bilateral substantia nigra hyperintensities (arrow) in T2-weighted images (F). Follow-up MRI (G–I) performed 4 months later shows the resolution of signal abnormalities and interval atrophy of the previously affected structures.
References
1. Murhekar M. Epidemiology of diphtheria in India, 1996–2016: implications for prevention and control. Am J Trop Med Hyg. 2017; 97:313–318.

2. Jayashree M, Shruthi N, Singhi S. Predictors of outcome in patients with diphtheria receiving intensive care. Indian Pediatr. 2006; 43:155–160.
3. Jhunjhunwala K, Netravathi M, Pal PK. Movement disorders of probable infectious origin. Ann Indian Acad Neurol. 2014; 17:292–297.

4. Das B, Chakravarthi S, Goyal MK, Modi M, Vyas S, Lal V, et al. Postencephalitic parkinsonism: interesting clinico-imaging correlation. J Neurol Sci. 2014; 343:215–217.

5. Mikulowski V. Encephalitis in diphtheria. Arch Fr Pediatr. 1956; 13:293–298.
7. Hall WE. Diphtheria and acute toxic encephalitis, with the report of a case. Can Med Assoc J. 1932; 26:566–569.
8. Foo JC, Rahmat K, Mumin NA, Koh MT, Gan CS, Ramli N, et al. Diphtheric encephalitis and brain neuroimaging features. J Clin Neurosci. 2017; 45:155–157.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2019.15.3.424.
Supplementary Video 1
Clinical video of the child in the fourth week of illness showing rigidity with dystonic posturing and mild dystonic tremors of the upper limbs. Video of the child at a 4-month follow-up showing an excellent outcome, with nearnormal gait and the absence of extrapyramidal symptoms. Higher mental functions were also almost normal with minimal memory disturbances.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download