INTRODUCTION
METHODS
Data source
Participants
Study measures
Table 1
Summary of the Myasthenia Gravis Foundation of America patient registry enrollment questions used in the study
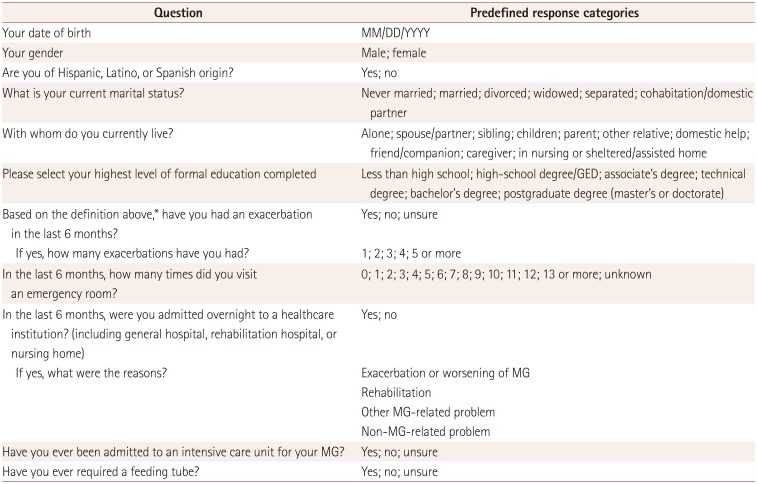
Reproduced with permission from the MGFA.
*Definition of an exacerbation of MG: development of new symptoms or worsening of existing symptoms that lasted >7 days and occurred ≥30 days after the last exacerbation. In an exacerbation, MG symptoms generally worsen over a period of days to weeks. They then improve over several weeks or months, usually with specific treatment. An exacerbation can be associated with several different symptoms that worsen simultaneously.
GED: general equivalency diploma, MG: myasthenia gravis.
Statistical analyses
RESULTS
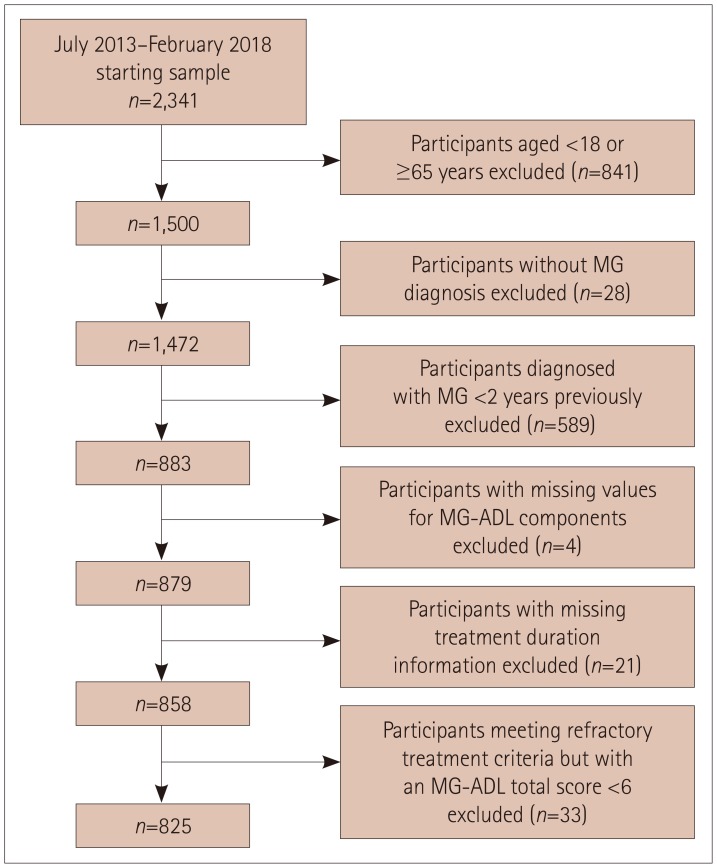 | Fig. 2Flow chart showing selection process for the study sample. MG-ADL: myasthenia gravis activities of daily living scale. |
Table 2
Participant demographics
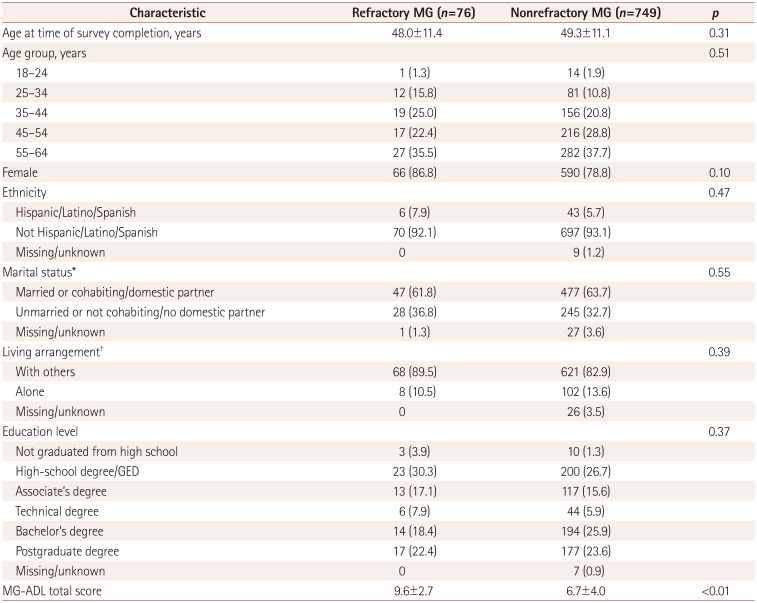
Data are mean±standard deviation or n (%) values. Frequency or mean values of study variables were compared between participants with refractory and nonrefractory MG using χ2 tests for categorical variables and t-tests for continuous variables. p values were calculated after excluding participants with unknown/missing data.
*Six predefined options, which were simplified into married or unmarried, †Ten predefined options, which were simplified into living alone or with others.
GED: general equivalency diploma, MG-ADL: myasthenia gravis activities of daily living scale.
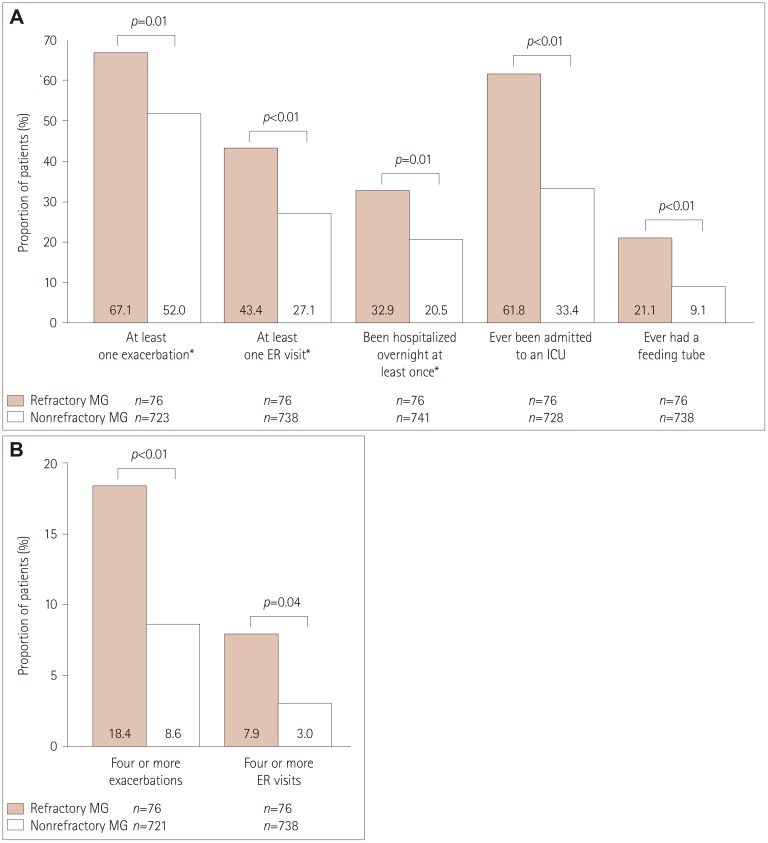 | Fig. 3Exacerbations in and healthcare resource utilization by participants with refractory MG and nonrefractory MG (A) and numbers of exacerbations and ER visits during the 6 months before enrollment in participants with refractory and nonrefractory MG (B). Frequencies of study variables were compared between participants with refractory and nonrefractory MG using χ2 tests for categorical variables and the number of exacerbations, and Fisher's exact test for the number of ER visits. p values are for comparisons between the refractory- and nonrefractory-MG groups, and were calculated after excluding data from participants with unknown/missing data. *During the 6 months before enrollment. ER: emergency room, ICU: intensive care unit, MG: myasthenia gravis. |
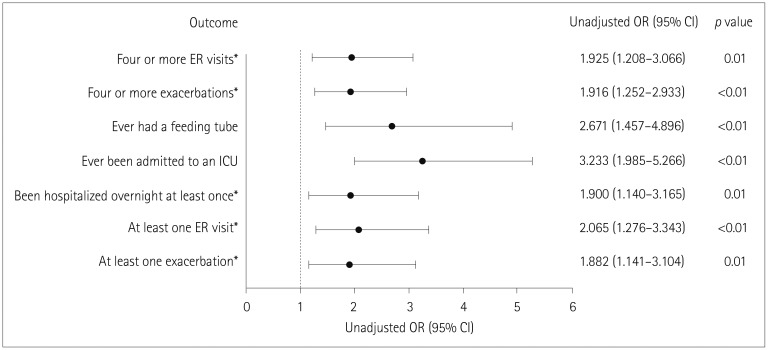 | Fig. 4Unadjusted ordinal logistic model examining the impact of refractory MG on exacerbations and healthcare resource utilization. *During the 6 months before enrollment. Analyses were based on fitting logistic models for binary outcomes except for when analyzing the numbers of exacerbations and ER visits, for which proportional-odds models for ordinal outcomes were fitted. CI: confidence interval, ER: emergency room, ICU: intensive care unit, MG: myasthenia gravis, OR: odds ratio. |
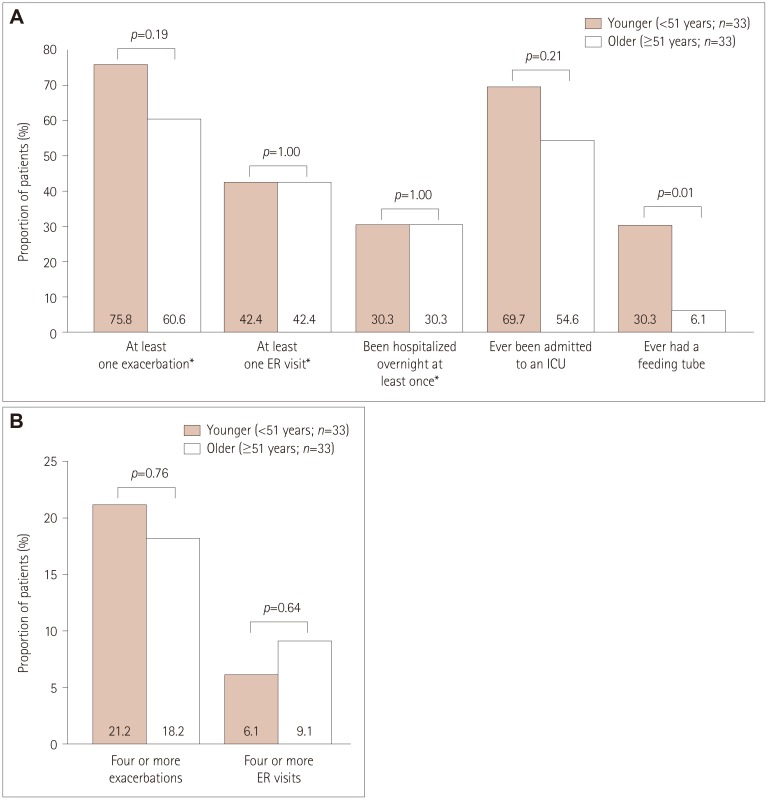 | Fig. 5Exacerbations in and healthcare resource utilization by younger and older female participants with refractory MG (A) and numbers of exacerbations and ER visits during the 6 months before enrollment in younger and older female participants with refractory MG (B). Frequencies of study variables were compared between participants with refractory and nonrefractory MG using χ2 tests for categorical variables and the number of exacerbations, and using Fisher's exact test for the number of ER visits. p values are for comparisons between the refractory- and nonrefractory-MG groups, and were calculated after excluding data from participants with unknown/missing data. *During the 6 months before enrollment. ER: emergency room, ICU: intensive care unit, MG: myasthenia gravis. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download