1. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014; 15:860–873. PMID:
25023924.
2. Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012; 16:987–1007. PMID:
22038683.

3. Högl B, Kiechl S, Willeit J, Saletu M, Frauscher B, Seppi K, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005; 64:1920–1924. PMID:
15955944.

4. Cho YW, Shin WC, Yun CH, Hong SB, Kim JH, Allen RP, et al. Epidemiology of restless legs syndrome in Korean adults. Sleep. 2008; 31:219–223. PMID:
18274269.

5. Yang X, Liu B, Shen H, Li S, Zhao Q, An R, et al. Prevalence of restless legs syndrome in Parkinson's disease: a systematic review and meta-analysis of observational studies. Sleep Med. 2018; 43:40–46. PMID:
29482811.

6. Trenkwalder C, Allen R, Högl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology. 2016; 86:1336–1343. PMID:
26944272.
7. Shin HY, Youn J, Yoon WT, Kim JS, Cho JW. Restless legs syndrome in Korean patients with drug-naïve Parkinson's disease: a nation-wide study. Parkinsonism Relat Disord. 2013; 19:355–358. PMID:
23047004.

8. Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, et al. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord. 2015; 30:229–237. PMID:
25449044.
9. Oh YS, Kim JS, Park IS, Song IU, Son YM, Park JW, et al. Association between nocturnal/supine hypertension and restless legs syndrome in patients with Parkinson's disease. J Neurol Sci. 2014; 344:186–189. PMID:
25016570.

10. Piao YS, Lian TH, Hu Y, Zuo LJ, Guo P, Yu SY, et al. Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep. 2017; 7:10547. PMID:
28874701.

11. Rana AQ, Siddiqui I, Mosabbir A, Athar A, Syed O, Jesudasan M, et al. Association of pain, Parkinson's disease, and restless legs syndrome. J Neurol Sci. 2013; 327:32–34. PMID:
23481589.

12. Lee JE, Shin HW, Kim KS, Sohn YH. Factors contributing to the development of restless legs syndrome in patients with Parkinson disease. Mov Disord. 2009; 24:579–582. PMID:
19097179.

13. Chahine LM, Ahmed A, Sun Z. Effects of STN DBS for Parkinson's disease on restless legs syndrome and other sleep-related measures. Parkinsonism Relat Disord. 2011; 17:208–211. PMID:
21216651.

14. Rijsman RM, Schoolderman LF, Rundervoort RS, Louter M. Restless legs syndrome in Parkinson's disease. Parkinsonism Relat Disord. 2014; 20(Suppl 1):S5–S9. PMID:
24262188.

15. Azmin S, Khairul Anuar AM, Nafisah WY, Tan HJ, Raymond AA, Hanita O, et al. Restless legs syndrome and its associated risk factors in Parkinson's disease. Parkinsons Dis. 2013; 2013:535613. PMID:
24455416.

16. Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson's disease: a case-controlled study. Mov Disord. 2003; 18:181–185. PMID:
12539212.

17. Zhu XY, Liu Y, Zhang XJ, Yang WH, Feng Y, Ondo WG, et al. Clinical characteristics of leg restlessness in Parkinson's disease compared with idiopathic restless legs syndrome. J Neurol Sci. 2015; 357:109–114. PMID:
26189051.

18. Möller JC, Unger M, Stiasny-Kolster K, Oertel WH. Restless legs syndrome (RLS) and Parkinson's disease (PD)-related disorders or different entities? J Neurol Sci. 2010; 289:135–137. PMID:
19755200.

19. Fereshtehnejad SM, Shafieesabet M, Shahidi GA, Delbari A, Lökk J. Restless legs syndrome in patients with Parkinson's disease: a comparative study on prevalence, clinical characteristics, quality of life and nutritional status. Acta Neurol Scand. 2015; 131:211–218. PMID:
25263328.

20. Shneyder N, Adler CH, Hentz JG, Shill H, Caviness JN, Sabbagh MN, et al. Autonomic complaints in patients with restless legs syndrome. Sleep Med. 2013; 14:1413–1416. PMID:
24152795.

21. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009; 32:589–597. PMID:
19480225.

22. Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology. 2011; 77:1941–1946. PMID:
22076542.

23. Nomura T, Inoue Y, Miyake M, Yasui K, Nakashima K. Prevalence and clinical characteristics of restless legs syndrome in Japanese patients with Parkinson's disease. Mov Disord. 2006; 21:380–384. PMID:
16211604.

24. Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002; 59:421–424. PMID:
11890847.

25. Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Restless legs syndrome and leg motor restlessness in Parkinson's disease. Parkinsons Dis. 2015; 2015:490938. PMID:
26504610.

26. Marsh L. Depression and Parkinson's disease: current knowledge. Curr Neurol Neurosci Rep. 2013; 13:409. PMID:
24190780.

27. Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004; 63:2410–2412. PMID:
15623715.

28. Klepitskaya O, Liu Y, Sharma S, Sillau SH, Tsai J, Walters AS. Deep brain stimulation improves restless legs syndrome in patients with Parkinson disease. Neurology. 2018; 91:e1013–e1021. PMID:
30111549.

29. Liguori C, Mercuri NB, Stefani A, Pierantozzi M. Effective treatment of restless legs syndrome by safinamide in Parkinson's disease patients. Sleep Med. 2018; 41:113–114. PMID:
29268951.

30. Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep. 2014; 37:369–372. PMID:
24497665.

31. Szatmari S Jr, Bereczki D, Fornadi K, Kalantar-Zadeh K, Kovesdy CP, Molnar MZ. Association of restless legs syndrome with incident Parkinson's disease. Sleep. 2017; 40:zsw065.

32. Dragan EM, Chen Z, Ondo WG. Does idiopathic restless legs syndrome delay onset and reduce severity of Parkinson's disease: a pilot study. Int J Neurosci. 2015; 125:526–530. PMID:
25405536.

33. Moccia M, Erro R, Picillo M, Santangelo G, Spina E, Allocca R, et al. A four-year longitudinal study on restless legs syndrome in Parkinson disease. Sleep. 2016; 39:405–412. PMID:
26564123.

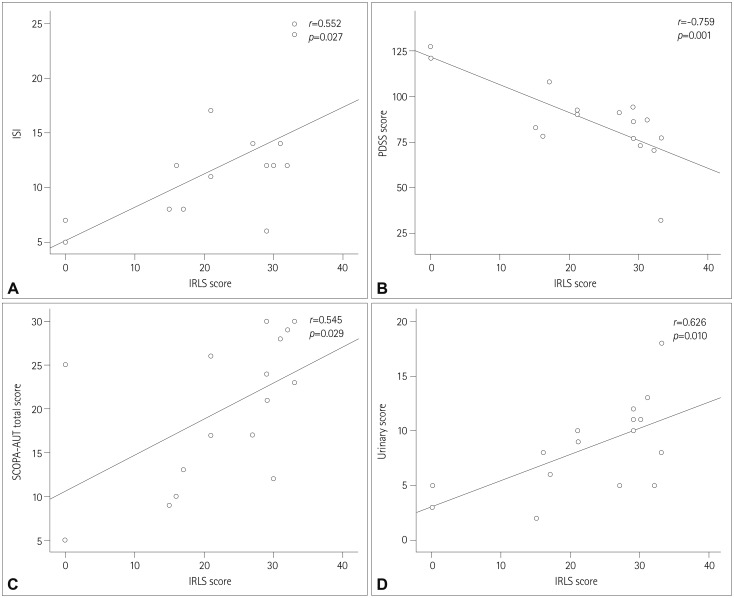
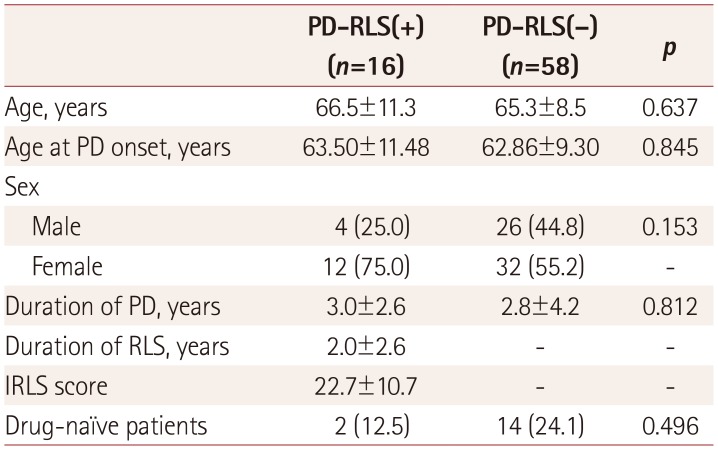
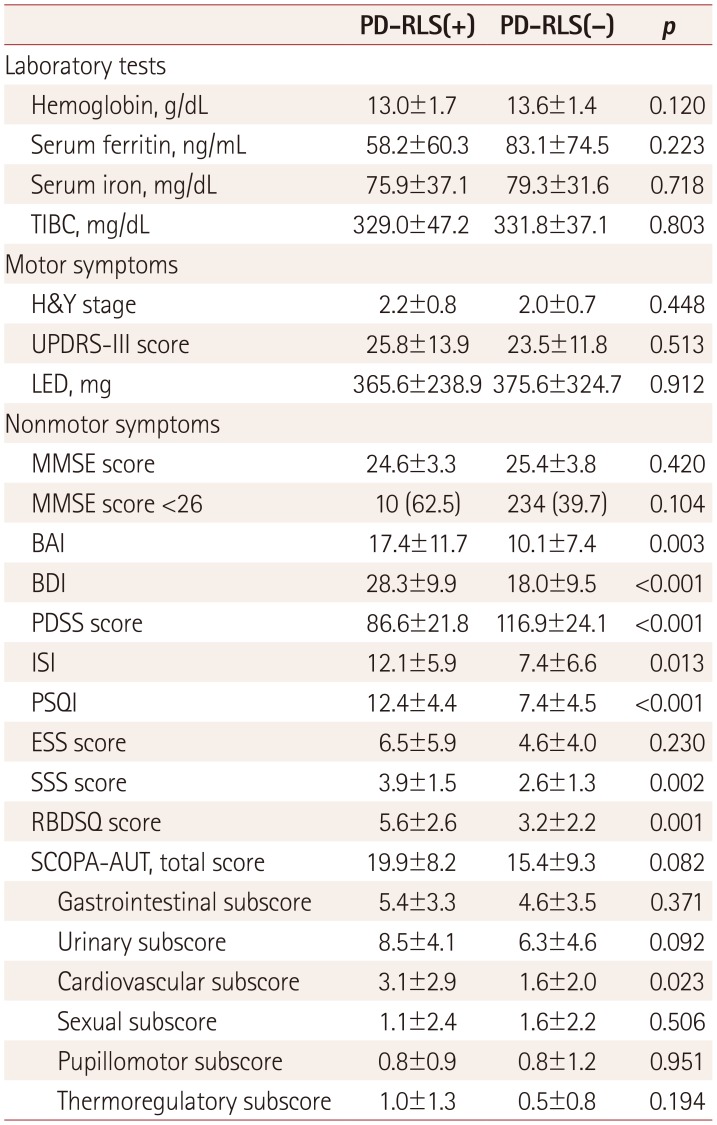
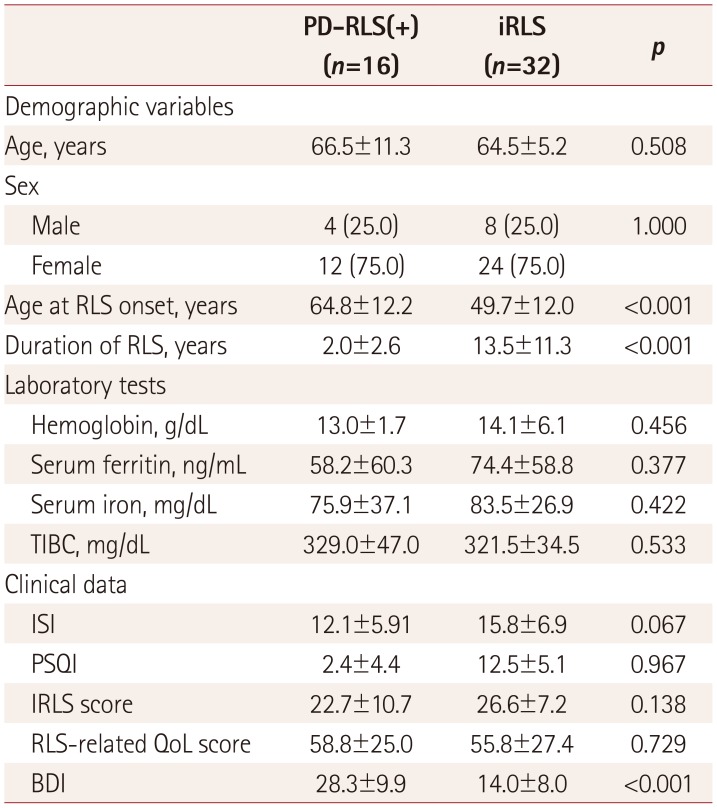




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download