1. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001; 345:311–318. PMID:
11484687.

2. Engel J Jr. The timing of surgical intervention for mesial temporal lobe epilepsy: a plan for a randomized clinical trial. Arch Neurol. 1999; 56:1338–1341. PMID:
10555652.
3. Al-Otaibi F, Baeesa SS, Parrent AG, Girvin JP, Steven D. Surgical techniques for the treatment of temporal lobe epilepsy. Epilepsy Res Treat. 2012; 2012:374848. PMID:
22957228.

4. Muzumdar D, Patil M, Goel A, Ravat S, Sawant N, Shah U. Mesial temporal lobe epilepsy–An overview of surgical techniques. Int J Surg. 2016; 36:411–419. PMID:
27773861.
5. Georgiadis I, Kapsalaki EZ, Fountas KN. Temporal lobe resective surgery for medically intractable epilepsy: a review of complications and side effects. Epilepsy Res Treat. 2013; 2013:752195. PMID:
24288602.

6. Kim SM, Kim SH, Seo DW, Lee KW. Intraoperative neurophysiologic monitoring: basic principles and recent update. J Korean Med Sci. 2013; 28:1261–1269. PMID:
24015028.

7. Ichikawa T, Suzuki K, Sasaki T, Matsumoto M, Sakuma J, Oinuma M, et al. Utility and the limit of motor evoked potential monitoring for preventing complications in surgery for cerebral arteriovenous malformation. Neurosurgery. 2010; 67(3 Suppl Operative):ons222–ons228. PMID:
20679926.

8. Maruta Y, Fujii M, Imoto H, Nomura S, Tanaka N, Inamura A, et al. Strategies and pitfalls of motor-evoked potential monitoring during supratentorial aneurysm surgery. J Stroke Cerebrovasc Dis. 2016; 25:484–495. PMID:
26639401.

9. Neuloh G, Pechstein U, Cedzich C, Schramm J. Motor evoked potential monitoring with supratentorial surgery. Neurosurgery. 2007; 61(1 Suppl):337–346. PMID:
18813156.

10. Szelényi A, Hattingen E, Weidauer S, Seifert V, Ziemann U. Intraoperative motor evoked potential alteration in intracranial tumor surgery and its relation to signal alteration in postoperative magnetic resonance imaging. Neurosurgery. 2010; 67:302–313. PMID:
20644415.

11. Mathon B, Navarro V, Bielle F, Nguyen-Michel VH, Carpentier A, Baulac M, et al. Complications after surgery for mesial temporal lobe epilepsy associated with hippocampal sclerosis. World Neurosurg. 2017; 102:639–650.e2. PMID:
28389411.

12. Girvin JP. Resection of intracranial lesions under local anesthesia. Int Anesthesiol Clin. 1986; 24:133–155.

13. Schuele SU, Lüders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008; 7:514–524. PMID:
18485315.

14. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008; 7:525–537. PMID:
18485316.

15. Erba G, Winston KR, Adler JR, Welch K, Ziegler R, Hornig GW. Temporal lobectomy for complex partial seizures that began in childhood. Surg Neurol. 1992; 38:424–432. PMID:
1298107.

16. Heller AC, Padilla RV, Mamelak AN. Complications of epilepsy surgery in the first 8 years after neurosurgical training. Surg Neurol. 2009; 71:631–637. PMID:
18514273.

17. Oertel J, Gaab MR, Runge U, Schroeder HW, Wagner W, Piek J. Neuronavigation and complication rate in epilepsy surgery. Neurosurg Rev. 2004; 27:214–217. PMID:
15048558.

18. Kombos T, Picht T, Derdilopoulos A, Suess O. Impact of intraoperative neurophysiological monitoring on surgery of high-grade gliomas. J Clin Neurophysiol. 2009; 26:422–425. PMID:
19952567.

19. Talacchi A, Turazzi S, Locatelli F, Sala F, Beltramello A, Alessandrini F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010; 100:417–426. PMID:
20467787.

20. Zhong J, Dujovny M, Perlin AR, Perez-Arjona E, Park HK, Diaz FG. Brain retraction injury. Neurol Res. 2003; 25:831–838. PMID:
14669526.

21. Lyon R, Feiner J, Lieberman JA. Progressive suppression of motor evoked potentials during general anesthesia: the phenomenon of “anesthetic fade”. J Neurosurg Anesthesiol. 2005; 17:13–19. PMID:
15632537.
22. Abboud T, Huckhagel T, Stork JH, Hamel W, Schwarz C, Vettorazzi E, et al. Why does threshold level change in transcranial motor-evoked potentials during surgery for supratentorial lesions? J Neurosurg Anesthesiol. 2017; 29:393–399. PMID:
27482981.

23. MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002; 19:416–429. PMID:
12477987.

24. Legatt AD, Emerson RG, Epstein CM, MacDonald DB, Deletis V, Bravo RJ, et al. ACNS guideline: transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2016; 33:42–50. PMID:
26756258.
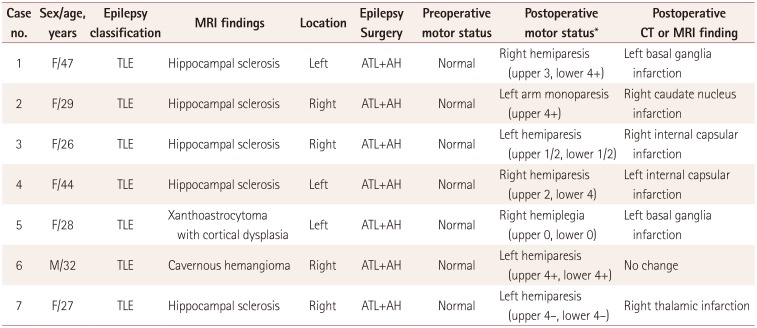
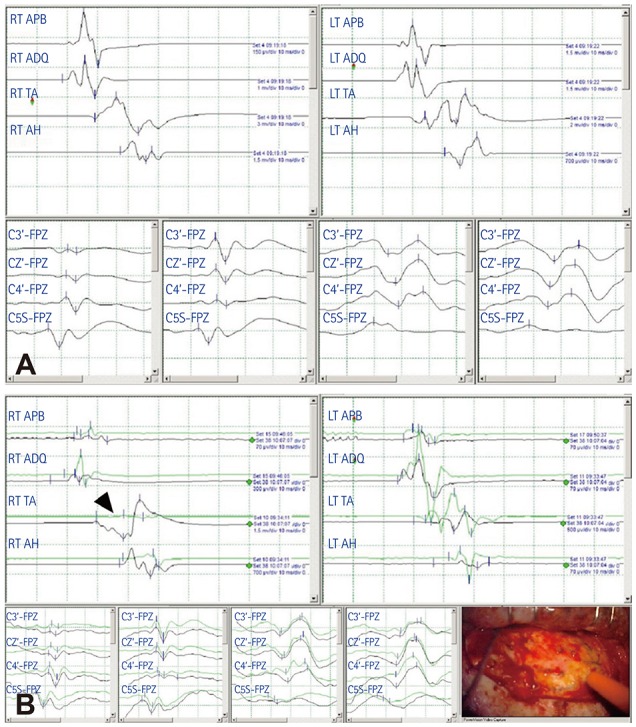
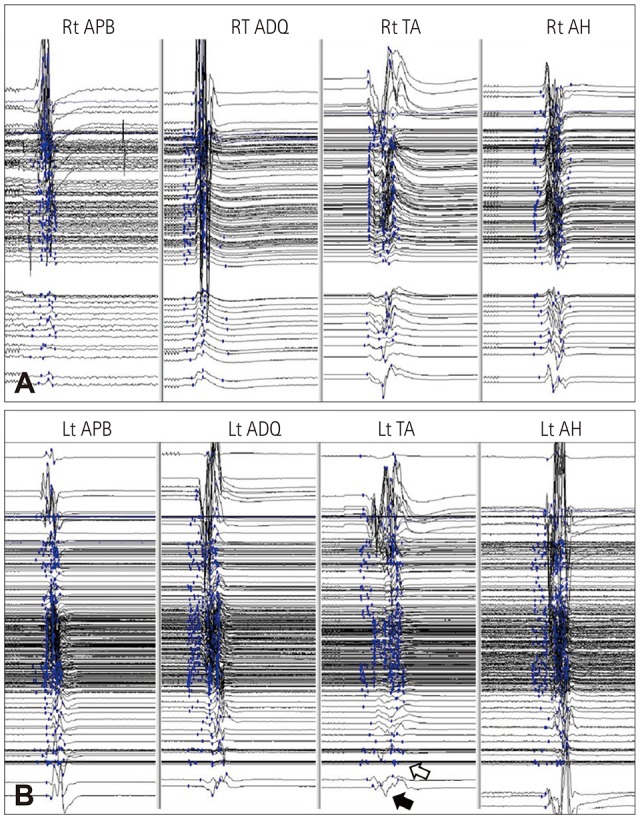
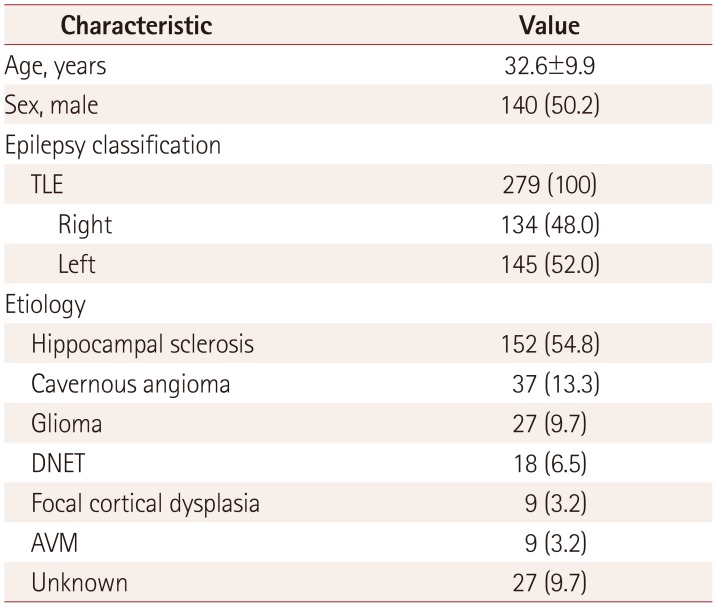




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download