This article has been
cited by other articles in ScienceCentral.
Abstract
Esophageal atresia (EA) is a diverse disease entity. We present a case of long gap EA without fistula corrected through totally laparoscopic and thoracoscopic esophageal replacement using gastric tube. A male baby weighing 3,000 g, with suspicion of EA, was born at gestational age of 37+6 weeks. Gastrostomy was made at an age of two days; seven months later, definite operation was planned. We determined to perform the gastric tube replacement due to long gap revealed by fluoroscopy. Gastric mobilization, gastric tube formation, and pyloroplasty were performed laparoscopically. An isoperistaltic 9 cm gastric tube was made using 2 Endo GIA 45, and interrupted end-to-end esophago-esophagostomy was performed thoracoscopically. With laparoscopy, gastropexy to the diaphragm was performed through the interrupted suture. Operation time was 370 minutes; there was no intraoperative event. Postoperative course was uneventful. He underwent esophageal balloon dilatation due to anastomosis stenosis in the months after surgery.
Keywords: Esophageal atresia, Laparoscopy, Thoracoscopy, Minimally invasive surgical procedures
INTRODUCTION
Long-gap esophageal atresia (LGEA) usually occurs as Gross type A, which constitutes only 4% of all EA patients [
1]. The rarity of these patients may partially contribute to the lack of evidence establishing definite LGEA correction procedures. Serial esophageal elongation, colon or intestinal interposition, gastric pull-up (GPU), and gastric tube formation have been attempted to correct LGEA. Since the first successful thoracoscopic repair (TR) in EA without tracheoesophageal fistula (TEF) in 1999, TR became a standard procedure in many institutions [
23]. TR was applied in the operation of EA with TEF in 2000 and became widely performed for patients with relatively stable conditions [
3]. However, minimally invasive surgery (MIS) is hard to apply in LGEA due to the complexity of procedures.
We introduce a case of LGEA, corrected by esophageal replacement with gastric tube through totally laparoscopic and thoracoscopic surgery.
CASE REPORT
A male baby weighing 3,000 g, with prenatal suspicion of EA was born at gestational age of 37+6 weeks. Initial infantogram at birth revealed no bowel gas and a feeding tube in the proximal esophageal pouch, suggesting EA Gross type A, specifically LGEA. Further evaluations including brain, spinal, abdominal ultrasonography and echocardiography did not show any other major anomalies. Gastrostomy was made 2 days after birth. He was admitted once to hospital for viral pneumonia (pathogen: respiratory syncytial virus) at 4 months, and otherwise healthy before definite operation.
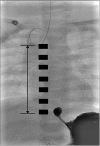
Seven months later, his body weight was 11.2 kg, and definite operation was planned. Since the distance measured via fluoroscopy between the upper and lower esophageal stumps was more than 6 vertebrae (i.e. too long to achieve primary end-to-end anastomosis), esophageal replacement with gastric tube (
Fig. 1) was performed.
Fig. 1
Fluoroscopy at the age of 7 months. Rudimentary distal esophagus, 6 vertebrae gap, distance from proximal to distal atresia: 8 cm.
1. Surgical technique
The operation was performed in 3 steps: laparoscopy, thoracoscopy, and a second laparoscopy. Under general anesthesia, the patient was positioned supine. The first 5 mm port was inserted using the open technique below the umbilicus. A pneumoperitoneum was maintained with pressure 10 mmHg, flow 1 L/min. Two 12 mm working ports were placed in bilateral upper abdomen and a 3 mm port was placed in the right lower quadrant (
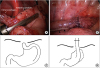
Fig. 2A). Gastrostomy was closed with intracorporeal interrupted 4–0 absorbable sutures. To clarify esophageal hiatus anatomy, the liver was dissected and retracted with snake retractor through a right upper quadrant port. We planned to make a gastric tube to avoid extensive dissection. The short gastric artery and left gastroepiploic artery were the only dissected and ligated. We made an isoperistaltic 9 cm gastric tube with two Endo GIA (Covidien, Mansfield, MA, USA) 45 along with greater curvature, conserving the distal esophagus (
Fig. 3A). The gastric tube was placed in the thoracic cavity through the esophageal hiatus. We performed Heineke-Mikulicz pyloroplasty with interrupted 4–0 absorbable sutures.
Fig. 2
Port location for (A) laparoscopic procedure and (B) thoracoscopic procedure.

Fig. 3
Isoperistaltic gastric tube formation using 2 Endo GIA 45 (Covidien, Mansfield, MA, USA) along with greater curvature. Hand sewn end-to-end anastomosis, interrupted 5-0 Vicryl suture. (A) Gastric tube (stapler line annotated), (B) Proximal esophagus, (C) Diagram before reconstruction, and (D) Final position of stomach, diaphragm, and esophagus.
The patient was then positioned left laterally. Three 5 mm ports were inserted in the standard locations for thoracoscopic esophageal atresia repair (
Fig. 2B). A pneumothorax was maintained with pressure 5 mmHg, flow 1 L/min. The proximal esophageal pouch was dissected and hand-sewn, interrupted, end-to-end esophago-esophagostomy was done using 5–0 absorbable sutures with the distal esophagus left in gastric tube (
Fig. 3B). Through one of 5 mm ports, a chest tube was inserted and closed, and aseptic dressing was applied. Diagram regarding reconstruction was shown in
Fig. 3C and D.
The patient was positioned supine again and laparoscopic procedure was resumed. The esophageal hiatus was inspected and the stomach was found to be appropriately positioned. Gastropexy to the diaphragm was performed through the interrupted sutures. Operation time was 370 minutes, and there was no intraoperative event.
2. Postoperative course
Immediate postoperative course was uneventful. He was discharged eating orally. During first 4 months after operation, he underwent esophageal dilatation 3 times due to anastomosis site stenosis. We regularly follow up performing esophageal dilatation.
DISCUSSION
The definition of LGEA has no global consensus. Some centers use a definite distance [
1] while others define LGEA functionally as the inability to perform a primary anastomosis because of the long gap [
4]. Thus, comparison between studies is difficult and ideal management remains unclear. Although primary anastomosis with native esophagus demonstrates better management than GPU [
5], tension-free anastomosis is not always possible. Anastomotic stricture, one of the most common complications after correction of EA, is closely related to anastomotic tension [
6]. Esophageal replacement with colon, jejunum or stomach is a good option for tension-free anastomosis. GPU is generally accepted as the best replacement procedure among those previously mentioned. GPU does not need bowel preparation and has only one anastomosis compared to jejunal interposition or colon interposition. However, high respiratory complication and leakage rates are reported following GPU, likely due to the relatively large stomach occupying a narrow thoracic cavity and extensive gastric vessel ligation for obtaining gastric mobilization to perform GPU [
5]. To overcome these drawbacks, one institution reported long-term results of partial gastric pull-up (PGP) [
7]. The incidence of stenosis and gastro-esophageal reflux after PGP is considerably high, even compared with alternative surgical techniques for the treatment of LGEA and their high complication rates. Many disadvantages of open thoracotomy have been reported such as air leak, postoperative pain, significant mucoskeletal morbidity [
3].
To overcome potential problems previously mentioned, we performed this minimally invasive procedure. Thoracoscopy is a comparable, even superior, operative modality than thoracotomy with respect to pain, stress, and musculoskeletal morbidities [
8]. Without cervical esophagostomy, which may be accidentally detrimental to mediastinal structures, we performed gastric tube pull-up and anastomosis using both laparoscopy and thoracoscopy. Minimal dissection preserving left and right gastric and right gastroepiploic artery to the stomach maintains sufficient blood flow. We made a gastric tube for minimizing the volume effect of the stomach to avoid respiratory complications. Parilli et al. [
9] reported laparoscopic transhiatal esophagectomy and GPU in LGEA, however those investigators included extracorporeal esophagectomy and anastomosis. As far as we know, this is the first reported case of this procedure.
Recently, Elfiky et al. [
10] reported the results of 50 patients who underwent gastric tube for esophagoplasty. Long-term follow-up reports of weight gain, swallowing pattern, quality of life, and overall satisfaction were excellent. Yet, open laparotomy plus cervical esophagostomy was performed. This leaves the question of the long-term synergetic results of the previous study plus the MIS presented herein.
We presented a successful gastric tube replacement for the LGEA through minimally invasive procedure. A prospective comparison study is needed to evaluate the long-term outcomes of this procedure.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download