Abstract
Background and Purpose
Methods
Results
Acknowledgements
References
Supplementary Materials
Supplementary Fig. 1
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Fig. 1
Disease-related metabolic patterns in the various study groups. A: The de novo Parkinson's disease with probable RBD-PDRBD-RP identified by network analysis of 18F-FDG PET scans in 21 PDRBD patients and 24 age- and gender-matched HC. Voxels with warm colors represent positive regions (metabolic increases) and those with cool colors are negative regions (metabolic decreases). B: The PDRBD-RP differed significantly among the groups, as determined by one-way ANOVA followed by post-hoc Tukey's HSD tests. The PDRBD-RP increased gradually from HC to iRBD and then to PDRBD. C: The RBD-RP identified by the network analysis of 18F-FDG PET scans of 28 iRBD patients and 24 age- and gender-matched HC. Voxels with warm colors represent positive regions (metabolic increases) and those with cool colors denote negative regions (metabolic decreases). D: The RBD-RP differed significantly among the groups, as determined by one-way ANOVA followed by post-hoc Tukey's HSD tests. The pattern was higher in both patient groups than in HC, with no difference between the two patient groups. 18F-FDG: 18F-fluorodeoxyglucose, GP: globus pallidus, HC: healthy controls, HSD: honestly significant difference, iRBD: idiopathic rapid eye movement sleep behavior disorder, PDRBD-RP: de novo Parkinson's disease with probable rapid eye movement sleep behavior disorder-related spatial covariance pattern, RBD: rapid eye movement sleep behavior disorder, RBD-RP: rapid eye movement sleep behavior disorder-related spatial covariance pattern, SMA: supplementary motor area.
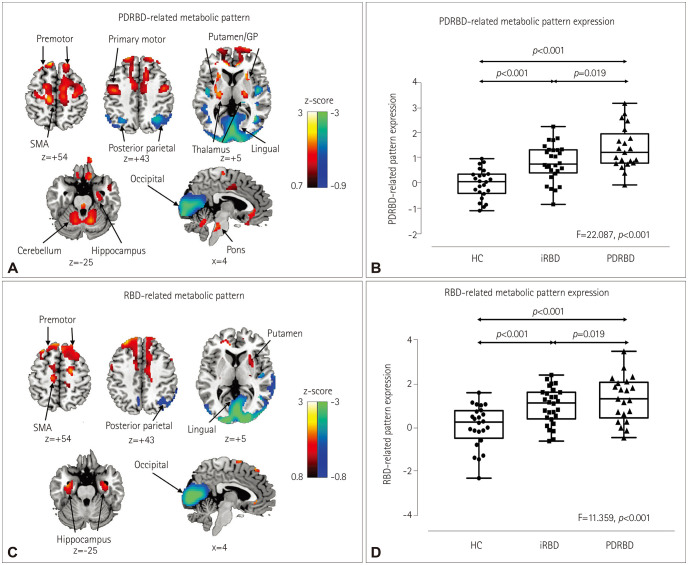
Fig. 2
Validation of the PDRBD-RP in a separate Parkinson's disease cohort with rapid eye movement sleep behavior disorder confirmed by polysomnography. Compared with HC, the validation PDRBD group showed a significantly increased PDRBD-RP that was similar to that in the de novo PDRBD group. HC: healthy controls, PDRBD-RP: de novo Parkinson's disease with probable rapid eye movement sleep behavior disorder-related spatial covariance pattern, ns: not significant.

Fig. 3
Correlations of nonmotor Parkinson's disease features in iRBD with the PDRBD-RP and RBD-RP. Correlations of the PDRBD-RP (A) and RBD-RP (D) with olfactory function (butanol threshold test). Comparisons of the PDRBD-RP (B) and RBD-RP (E) between the normosmic and anosmic iRBD patients. Correlations of the PDRBD-RP (C) and RBD-RP (F) with the reaction time z score in trail-making test B. The correlation analysis were performed using Pearson's correlation coefficient, and the group comparisons were performed using analysis of covariance, with age as the covariate. iRBD: idiopathic rapid eye movement sleep behavior disorder, PDRBD-RP: de novo Parkinson's disease with probable rapid eye movement sleep behavior disorder-related spatial covariance pattern, RBD-RP: rapid eye movement sleep behavior disorder-related spatial covariance pattern.

Table 1
Demographic and clinical characteristics of the participants included in the final analysis
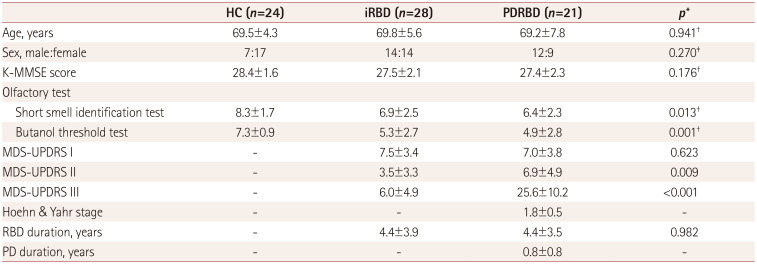
Data are mean±SD or n values.
*Independent t-test except where indicated otherwise, †One-way ANOVA, ‡Chi-square test.
HC: healthy controls, iRBD: idiopathic rapid eye movement sleep behavior disorder, K-MMSE: Korean version of the Mini Mental State Examination, MDS-UPDRS: Unified Parkinson's disease Ratings Scale as revised by the Movement Disorders Society Task Force, PD: Parkinson's disease, PDRBD: de novo Parkinson's disease with probable rapid eye movement sleep behavior disorder.
Table 2
Uptake of 18F-FP-CIT-nortropane in the three study groups
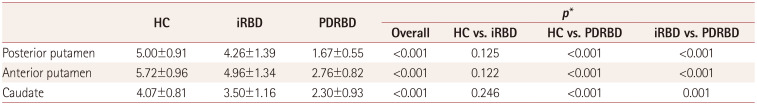
Data are mean±SD values.
*One-way ANOVA with post-hoc Tukey's HSD tests.
18F-FP-CIT: 18F-N-3-fluoropropyl-2β-carboxymethoxy-3β-(4-iodophenyl)-nortropane, HC: healthy controls, HSD: honestly significant difference, iRBD: idiopathic rapid eye movement sleep behavior disorder, PDRBD: de novo Parkinson's disease with probable rapid eye movement sleep behavior disorder.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download