Abstract
Although recent advances in molecular targeted therapy and immuno-oncology have revolutionized the landscape of lung cancer therapeutics, cytotoxic chemotherapy remains an essential component of lung cancer treatment. Extensive evidence has demonstrated the clinical benefit of chemotherapy, either alone or in combination with other treatment modalities, on survival and quality of life of patients with early and advanced lung cancer. Combinational approaches with other classes of anti-neoplastic agents and new drug-delivery systems have revealed promising data and are areas of active investigation. Chemotherapy is recommended as a standard of care in patients that have progressed after tyrosine kinase inhibitors or immune checkpoint inhibitors. Chemotherapy remains the fundamental means of lung cancer management and keeps expanding its clinical implication. This review will discuss the current position and future role of chemotherapy, and specific consideration for its clinical application in the era of precision medicine.
Lung cancer is the leading cause of cancer-related mortality worldwide. In 2012, 1,824,701 new cases were diagnosed, and 1,590,000 patients died of lung cancer globally1. Although epidemiologic data showed an increasing trend of survival rate of lung cancer, 88,655 patients died from lung cancer in Korea during the period 2008 to 20122. Numerous efforts have been made to reduce the mortality of this devastating disease, and we have noticed great progress in the management of lung cancer over the past decades.
Notably, molecular-targeted therapy, including epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) and anaplastic lymphoma kinase (ALK) inhibitors, has improved the survival of patients with tumors harboring driving genetic alterations3. In addition, immune checkpoint inhibitors (ICIs) targeting programmed death-l or its ligand (PD-L1) have also shown survival benefit in selected or unselected populations456, and are actively under clinical trials. However, only about 15% to 40% of all non-small cell lung cancer (NSCLC) patients have EGFR mutation or ALK rearrangement7, and only about 30% of the EGFR - and ALK-negative patients had tumors with PD-L1 tumor proportion score (TPS) of 50% or greater4, which means the rest of the patients are candidates for the first-line platinum-based chemotherapy. Moreover, in the cases of TKIs or ICIs failure, chemotherapy is recommended as a valid subsequent treatment8910. In this review, I will discuss current evidence of the clinical benefit of chemotherapy, new treatment strategies, and potential biomarkers after a brief review of the history of development of lung cancer chemotherapy.
Before the chemotherapy era, the median overall survival (OS) of metastatic lung cancer was merely 2 to 4 months with the best supportive care11. Although early generation chemotherapeutic agents, including methotrexate and doxorubicin, were used for the management of lung cancer in the 1970s, the clinical benefit was modest12. Substantial survival benefit in lung cancer patients was shown only after the development and introduction of platinum and new generation chemotherapeutic agents, including taxanes, vinorelbine, and gemcitabine, during the 1980s and 1990s. A landmark meta-analysis by the Non-Small Cell Lung Cancer Collaborative Group in 1995 has demonstrated that platinum-based chemotherapy significantly improved OS over the best supportive care (15% vs. 5% OS rate in 1 year)13. In addition, the platinum doublet showed superior efficacy over non-platinum based regimen or single agent14. Eastern Cooperative Oncology Group (ECOG) 1594 trial prospectively compared four platinum-based doublets for advanced NSCLC, and demonstrated that survival was not different among different regimens with median OS of 7.9 months, and good performance status (ECOG 0 or 1) was significantly associated with better survival15. According to this trial, combinations of platinum and those new generation chemotherapeutic agents have been recommended as the first-line treatment of advanced NSCLC, regardless of tumor histology.
In the early 2000s, pemetrexed was introduced as another new generation chemotherapeutic agent with proven efficacy in lung cancer. In the phase 3 JMDB trial, pemetrexed demonstrated pronounced clinical benefit on nonsquamous than squamous cell histology16, which provided the concept that histology does matter in the treatment of lung cancer. Subsequent phase 3 JMEN trial demonstrated the clinical benefit of pemetrexed maintenance treatment in nonsquamous cell carcinoma17. In that trial, the median OS was significantly higher in patients treated with pemetrexed maintenance after 4-cycled platinum doublet compared with those without maintenance therapy (13.4 months vs. 10.6 months; hazard ratio [HR], 0.79; p=0.012)17.
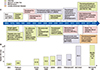
Anti-angiogenesis drugs such as bevacizumab, and new drug delivery systems such as nanoparticle albumin-bound paclitaxel (nab-paclitaxel) have also shown clinical benefit in terms of efficacy and tolerability in the middle 2000s and early 2010s, respectively, and have been approved as new valid agents for lung cancer treatment. In recent years, chemotherapy- based combinational approaches using TKIs or ICIs has shown survival benefit over chemotherapy alone1819. Figure 1 summarizes the evolution of chemotherapy-based therapeutic approaches and the progress of clinical benefit according to landmark trials.
Platinum compound indiscriminately attacks all rapidly dividing cells which results in drug-related side effects that include gastrointestinal toxicity, nephrotoxicity, neurotoxicity, ototoxicity, and myelosuppression20. Compared to cisplatin, carboplatin is slightly less associated with those toxicities except myelosuppression, and is also slightly less efficacious for the treatment of lung cancer20. In a meta-analysis, cisplatin-based chemotherapy showed a better objective response rate (ORR) (odds ratio [OR], 1.36; p<0.001) without OS benefit (HR, 1.050; p=0.515) compared with carboplatin-based chemotherapy21. However, subgroup analysis revealed that combinations of cisplatin and new generation agents yield 11% of OS benefit over carboplatin with the same new agents (HR, 1.106; p=0.039)21. In this analysis, cisplatin-based chemotherapy was more frequently associated with nausea and vomiting (OR, 2.51), but less associated with grade ≥3 thrombocytopenia (OR, 0.58)21. Based on the previous data, cisplatin combination should be a preferred regimen especially combined with new generation drugs. However, considering the modest OS benefit of cisplatin over carboplatin, and the purpose of chemotherapy in advanced disease where the goal is not cure but symptom and disease control, carboplatin is still a valid option. The choice of platinum compound in practice should be individualized based not only on the patients' age but also on comorbidities that may be associated with the risk of drugrelated adverse effects.
The ideal number of platinum-based chemotherapy cycles in patients with advanced NSCLC has long been a clinical issue2223. Guidelines recommend that patient with responsive or stable disease can continue to receive a total of 4 to 6 cycles of first-line systemic chemotherapy, and more than 6 cycles of chemotherapy is not recommended8910. A recent meta-analysis using individual patient data compared the survival of the patients who were treated with 3- or 4-cycled- versus 6-cycledfirst-line platinum doublet24. Modest median progression-free survival (PFS) increase in the group receiving 6 cycles of therapy compared to 3- or 4-cycled group (5.33 months vs. 6.09 months; HR, 0.79; p=0.0007) was observed, however, median OS was not different between the two groups (8.68 months vs. 9.54 months; HR, 0.94; p=0.33)24. Only 53% of patients completed the intended 6 cycles of chemotherapy, while 80% completed 3 or 4 treatment cycles in that analysis24. The survival data were similar, regardless of the platinum compounds used which is consistent with previous data15. Interestingly, the median OS was longer in patients treated with cisplatinbased doublet compared to those treated with carboplatin doublet (10.9 months and 7.0 months respectively, in patients with 3- or 4-cycled treatment)24, which confirmed the previous meta-analysis data21. Overall, OS difference could not be found between 3- or 4-cycled and 6-cycled platinum-doublet chemotherapy. However, the observations of increased PFS in patients receiving prolonged treatment, despite more frequent adverse events2324, have provided the fundamentals of the concept of ”maintenance therapy” beyond 4 or 6 cycles using less toxic agents. This concept was proved by several pivotal trials which will be discussed in the next section.
The main purpose of maintenance therapy is to improve survival by prolonging tumor response using a well-tolerated drug in patients who have not progressed during first-line treatments. At least one of the agents given in the first-line regimen can be continued, or a different agent that was not included in the first-line regimen can be chosen for maintenance treatment. The former represents continuation maintenance, while the latter represents switch maintenance. These approaches have now become a standard of care in patients with both squamous and nonsquamous NSCLC.
The pioneer in this new treatment strategy is pemetrexed. The clinical benefit of switch maintenance treatment using pemetrexed in patients with nonsquamous NSCLC patients was first demonstrated in the phase 3 JMEN trial17. The subsequent landmark phase 3 PARAMOUNT trial evaluated the role of pemetrexed as a continuation maintenance treatment in patients without progression after 4 cycles of pemetrexed/cisplatin25. Both PFS (4.1 months vs. 2.8 months; HR, 0.62; p<0.0001) and OS (13.9 months vs. 11.0 months; HR, 0.78; p=0.0195) were prolonged in the pemetrexed maintenance group compared to the placebo group2526. Based on the data of those data, this maintenance strategy was approved by Food and Drug Administration (FDA) at 2009. A subsequent analysis for patients' quality of life (QoL) during this trial demonstrated similar QoL profiles between both groups27.
In the aforementioned maintenance trials, the study subjects were enrolled regardless of EGFR mutational status. As pemetrexed-based doublet is not indicated as a first-line treatment in EGFR mutated patients, the clinical benefit of the pemetrexed maintenance treatment over the conventional chemotherapy needs to be re-evaluated for patients without EGFR mutations. A recent retrospective study reported survival data on those patients28. Interestingly, the median OS was significantly longer in the pemetrexed maintenance group (23.3 months vs. 11.0 months, p=0.005), and the OS difference was more pronounced compared with those of previous trials. Current guidelines recommend a continuation maintenance with bevacizumab, pemetrexed, bevacizumab plus pemetrexed (in nonsquamous histology), and gemcitabine (in squamous histology). Switch maintenance with pemetrexed is also recommended in nonsquamous histology. Currently, only pemetrexed continuation maintenance is reimbursed by the National Health Insurance System (NHIS) in Korea.
Despite chemotherapy has been successfully improved the survival of patients with lung cancer, there are several limitations of conventional chemotherapy: the high rate of adverse events due to nonspecific targeting, and relative short survival compared with targeted therapy or immunotherapy29. The drug delivery system has been a region of interest to overcome these drawbacks of conventional chemotherapy. This section will cover the two representative systems that are currently recommended or having shown promising data.
Nab-paclitaxel is an albumin-bound-stabilized nanoparticle formulation of paclitaxel designed to overcome the insolubility of the conventional solvent-based paclitaxel (sb-paclitaxel). Nab-paclitaxel has the merit that it requires a shorter infusion time (30 minutes vs. 3 hours), and can avoid hypersensitivity reactions, as it is free of Cremophor EL, a solvent for sb-paclitaxel30. While it may be less convenient in that nab-paclitaxel should be administered every week rather than every 3 weeks, it may be beneficial in terms of managing or avoiding critical adverse events.
A pivotal phase 3 trial has demonstrated significantly higher ORR and non-inferior PFS and OS of nab-paclitaxel/carboplatin compared to sb-paclitaxel combination31. Interestingly, the difference in ORR was more prominent in patients with squamous histology compared with nonsquamous histology. Moreover, the nab-paclitaxel arm was associated with a lower rate of grade ≥3 neutropenia, peripheral neuropathy, arthralgia, and myalgia, although it was associated with more grade ≥3 anemia and thrombocytopenia31. In a subsequent trial, nab-paclitaxel/carboplatin combination has shown OS benefit over sb-paclitaxel combination in elderly patients over 70 years old32. This survival benefit seen in elderly patients may be associated with less toxicity and more completion of preplanned treatment. In addition, the weekly dosing schedule of nab-paclitaxel might contribute to better treatment efficacy30. Current guidelines recommend nab-paclitaxel/carboplatin combination as a first-line regimen in both squamous and nonsquamous histology8910. In particular, the National Comprehensive Cancer Network (NCCN) guideline states that nab-paclitaxel may be substituted for either paclitaxel or docetaxel in patients who have experienced hypersensitivity reactions after receiving paclitaxel or docetaxel despite premedication, or where premedications are contraindicated10. In Korea, nab-paclitaxel use has been approved for pancreatic, breast and lung cancer treatment; however to date, its usage for lung cancer is not reimbursed by the NHIS. Given its favorable efficacy and tolerability, nab-paclitaxel is a valuable option for a first-line treatment in patients with advanced NSCLC.
Antibody drug conjugates (ADCs) are one of the targeted drug delivery systems. ADCs are complexes of antibody linked to a biologically active cytotoxic agent to be delivered specifically to cancer cells33. Two drugs worth mentioning in lung cancer are ado-trastuzumab emtansine for NSCLC, and rovalpituzumab tesirine for small cell lung cancer (SCLC). Adotrastuzumab emtansine is the FDA-approved ADC for human epidermal growth factor receptor 2 (HER2)-positive breast cancer34. In a recent phase 2 basket trial which included heavily treated HER2-mutant NSCLC patients, ado-trastuzumab emtansine showed 44% of response rate (RR), and 5 months of median PFS35. Toxicities were manageable, and no patient stopped therapy as a result of toxicity35.
Rovalpituzumab tesirine (Rova-T) is an ADC directed against ligand delta-like protein 3 (DLL3), a novel target expressed in patients with SCLC36. The first phase 1 trial evaluating Rova-T in patients with relapsed or refractory SCLC has reported impressive data: 18% of ORR and 68% of disease control rate (DCR)37. Intriguingly, high DLL3 expression was associated with high DCR; 88% and 50% in high (>50%) and low (<50%) DLL3 groups, respectively. In addition, high DLL3 group showed a significantly longer median PFS (4.3 months) and OS (5.8 months), compared with the overall population37. Rova-T showed a unique toxicity profile, notable for thrombocytopenia, serosal effusions, and skin reactions. The mechanism of these toxic effects is not clear, but probably relates to the pyrrolobenzodiazepine dimer component of this complex37. Overall, Rova-T showed encouraging single-agent antitumor activity with a manageable safety profile in patients with relapsed or refractory SCLC. In addition, DLL3 may be a potential predictive marker in this setting. Based on these promising data, several clinical trials investigating Rova-T in SCLC are now ongoing; a phase 3 trial on Rova-T maintenance treatment after 4-cycled platinum-based doublet, and another phase 3 trial comparing Rova-T and topotecan as second-line treatment for relapsed disease38.
As a monoclonal antibody that binds vascular endothelial growth factor (VEGF)-A, bevacizumab inhibits endothelial cell proliferation and migration which are the critical steps in carcinogenesis. Clinical efficacies of bevacizumab in lung cancer have been proved in several previous trials394041. In the phase 3 ECOG4599 trial, bevacizumab (at a dose of 15 mg/kg) combined with paclitaxel/carboplatin has demonstrated significantly clinical benefit in terms of RR (35% vs. 15%, p<0.001), PFS (6.2 months vs. 4.5 months, p<0.001), and OS (12.3 months vs. 10.3 months, p=0.003), compared to paclitaxel/ carboplatin alone39. In Europe, the phase 3 AVAiL trial attempted to confirm the benefit of bevacizumab in combination with cisplatin/gemcitabine at two different doses (7.5 mg/kg and 15 mg/kg)40. Although significantly better PFS in both doses of bevacizumab was shown, the OS was not different between the two groups42. The subsequent phase 3 BEYOND trial evaluating the efficacy of the bevacizumab (15 mg/kg) plus carboplatin/paclitaxel combination in a Chinese cohort has demonstrated significant improvements in both PFS (9.2 months vs. 6.5 months; HR, 0.40; p<0.001) and OS (24.3 months vs. 17.7 months; HR, 0.68; p=0.0154)41. A metaanalysis also demonstrated significant RR, PFS, and OS benefit of bevacizumab plus platinum-doublet over platinum-doublet alone43. As bevacizumab was associated with a high rate of life-threatening hemoptysis, tumor adjunct to major blood vessels, cavitary tumors and squamous histology are contraindicated, and bevacizumab plus platinum-doublet is recommended only for nonsquamous histology813. This combinational approach has been evaluated in adjuvant setting for surgically resected, early-staged NSCLC, however, it failed to prove OS benefit44.
Ramucirumab, a monoclonal antibody to VEGF receptor 2, and nintedanib, a multiple kinase inhibitor, are another angiogenesis inhibitors with proven survival benefit in the second or later line setting in lung cancer treatment4546. The phase 3 REVEL trial compared ramucirumab plus docetaxel combination and docetaxel alone in the second-line setting45. Of note in this trial, patients who previously received bevaciuzumab and those who had squamous histology were not excluded. Modest but statistically significant improvement in OS (10.5 months vs. 9.1 months; HR, 0.86; p=0.023) and PFS (4.5 months vs. 3.0 months; HR, 0.76; p<0.0001) was shown in combination arm45. Although, the NCCN guideline recommend use of ramucirumab plus docetaxel combination10, the use of the regimen seems to have not been widely adopted, maybe due to the concern of toxicities and relatively low cost-effectiveness47. Currently, bevacizumab and platinum doublet combination regimen is approved for the treatment of advanced nonsquamous NSCLC; however, this regimen is not covered by the NHIS in Korea.
Although molecular targeted therapy has dramatically improved the survival of EGFR-mutated or ALK-rearranged NSCLC patients48, it is undeniable that OS benefit is partly attributable to the use of subsequent chemotherapy after TKI failure. From this point of view, the combination of TKI and chemotherapy as a first-line treatment for advanced NSCLC is a feasible approach. The INTACT 1 trial investigated gefitinib plus gemcitabine/cisplatin combination in advanced NSCLC49. Although there were no significant unexpected adverse events, there was also no clinical benefit in the combination group49. Another phase 3 trial testing erlotinib plus gemcitabine/cisplatin also failed to prove clinical benefit over the control group50. In this trial, in only a small never-smoker subgroup, OS and PFS were increased in combination arm50.
FASTACT-2 trial has firstly demonstrated the potential benefit of the chemotherapy plus EGFR-TKI combination in advanced NSCLC18. In this trial, an intercalated combination of chemotherapy and erlotinib (150 mg/day on days 15–28) was associated with better PFS (16.8 months vs. 6.9 months; HR, 0.25; p<0.0001) and better OS (31.4 months vs. 20.6 months; HR, 0.48; p=0.0092) over chemotherapy alone in EGFR-mutated patients18. The safety profile was similar between the two groups18. Despite these positive findings, erlotinib plus chemotherapy combination has not yet been recommended in guidelines, maybe due to the issues of dose schedule, optimal intercalation, and post-study treatments in the trial51. The clinical benefit of this kind of combinational approach seems to be validated by further trials.
Until recently, potential antagonism between chemotherapy and immunotherapy is one of the concerns when considering the chemotherapy and ICI combination. However, there have been a bunch of studies that have supported optimistic data on this kind of combinational strategy. Chemotherapy can modulate tumor to be more susceptible for ICI by releasing tumor-specific antigens, up-regulation of major histocompatibility complex expression, increasing the cytotoxic lymphocyte to regulatory T-cell ratio, and inhibiting myeloid-derived suppressor cells52535455. Preclinical studies have also shown a synergistic effect of the chemotherapy and ICI combination5657. The phase 2 KEYNOTE-021G trial is a proof-of-concept study that demonstrated the superiority of combination of chemotherapy and immunotherapy in advanced NSCLC58. In this trial, pemetrexed/carboplatin combined with pembrolizumab showed significantly better RR and longer PFS than chemotherapy alone in previously untreated patients with metastatic nonsquamous NSCLC without EGFR or ALK genomic aberrations irrespective of PD-L1 expression58. Based on those data, this combination has been granted accelerated approval by the U.S. FDA in May 2017.
The clinical benefit of chemotherapy and ICI combination in the same clinical setting were investigated in the phase 3 KEYNOTE-189 trial in which pemetrexed maintenance treatment was permitted in the control arm19. Pembrolizumab in combination with pemetrexed/carboplatin showed significant improvement in both PFS (8.8 months vs. 4.9 months; HR, 0.52; p<0.001) and OS rate at 12 months (69.2% vs. 49.4%; HR, 0.49; p<0.001)19. In addition, the clinical benefit was found in all subgroups examined, including those with PD-L1 TPS of less than 1%19. Considering 41.3% of the patients in the placebo-combination group received ICIs on progression, the HR is quite impressive. Adverse events of grade 3 or higher occurred similarly in both groups (67.2% in the pembrolizumabcombination group and 65.8% in the placebo-combination group), although discontinuation due to adverse event was higher in the pembrolizumab-combination group (13.8%) than in the placebo-combination group (7.9%)19. The addition of pembrolizumab did not appear to increase the frequency of adverse events that are commonly associated with pemetrexed/carboplatin. Similarly, the incidence of most immune-mediated adverse events was not higher with the pembrolizumab-combination group than that previously observed with pembrolizumab monotherapy4. In addition, the interim data of KEYNOTE-407 phase 3 trial (NCT02775435) also demonstrated better response and longer PFS and OS in the pembrolizumab plus carboplatin/paclitaxel (or nab-paclitaxel) combination over chemotherapy alone in patients with metastatic squamous cell carcinoma irrespective of PD-L1 expression38. Taken together, “chemoimmunotherapy” may be a new standard of care for the first-line treatment of advanced NSCLC with manageable safety profile. The results of ongoing trials on the chemoimmunotherapy for NSCLC are eagerly awaited38.
The survival benefit of adjuvant chemotherapy for earlystaged resected lung cancers has been demonstrated in many previous trials59606162. A recent meta-analysis has clearly shown the benefit of chemotherapy after surgical resection (HR, 0.86; p<0.0001) with an absolute increase in survival of 4% at 5 years63. The benefit was consistent regardless of chemotherapy regimen or patient subgroups. In patients with T2ab-N0 tumors, adjuvant chemotherapy can be considered in selected patients with high-risk features including poorly differentiated tumors, 4 cm or larger tumor size, visceral pleural involvement, vascular invasion, wedge resection, and unknown lymph node status64.
In patients with completely resected N2 disease, postoperative chemoradiation has provided a survival advantage when compared with chemotherapy alone65. Postoperative chemoradiation can be either concurrent or sequential depending on the type of resection and lymph node status. The NCCN guideline recommends concurrent chemoradiation for R2 resection, whereas it recommends either sequential or concurrent chemoradiation for R1 resections10. Chemoradiation has also proved its survival benefit for unresectable stage III NSCLC over radiotherapy alone6667. Of note, concurrent chemoradiation showed superiority compared to sequential therapy in this setting (16% vs. 10% in 5-year-survival rate, p=0.046)68. The recommended regimens for chemoradiation are etoposide/cisplatin, vinblastine/cisplatin, paclitaxel/carboplatin (for both squamous and nonsquamous NSCLC), and pemetrexed/cisplatin or pemetrexed/carboplatin (for nonsquamous NSCLC). The phase 3 PROCLAIM trial compared the efficacy and safety of pemetrexed/cisplatin and etoposide/cisplatin followed by consolidation chemotherapy in patients with unresectable stage III nonsquamous NSCLC69. Both regimens were similar in terms of survival but the pemetrexed/cisplatin regimen was associated with less neutropenia (24.4% vs. 44.5%, p<0.001), and fewer grade 3 or higher adverse events (64.0% vs. 76.8%, p=0.001). These data suggest that pemetrexed/cisplatin is a valid option for concurrent chemoradiation in this setting.
The discovery and clinical application of biomarkers is critical not only for early detection of the disease but also for the identification of those patients expected to show the best response to therapy. Despite numerous studies, no biomarker to date has been found to have clinical significance for the prediction of outcomes after platinum-based chemotherapy. The KRAS mutation, although it has shown to be related to the poor survival of NSCLC patients in several studies7071, does not appear to be predictive of chemotherapeutic efficacy7273. Excision repair cross-complementation group 1 (ERCC1) has been linked with resistance to platinum-based chemotherapy in NSCLC7475. Low ERCC1 levels are related to increased recurrence in untreated patients after lung resection and are correlated with the prolonged survival of NSCLC patients treated with platinum-based adjuvant and palliative chemotherapy767778. However, ERCC1-tailored chemotherapy failed to prove its utility in two prospective randomized trials7980. Among other potential biomarkers, including ribonucleotide reductase M1, breast cancer 1, class III beta-tubulin, Bax and Fas, none was proven to be useful for the prediction of response or survival in platinum-based chemotherapy8182.
Thymidylate synthase (TS) is the primary target of pemetrexed and its expression has been linked with response to antifolate treatment in various cancers, including gastric, esophageal, and colorectal cancers838485. In NSCLC, high TS protein expression is associated with poor prognosis following lung resection8687 and high TS mRNA level is associated with poor response to neoadjuvant pemetrexed/gemcitabine treatment88. In the phase 3 JMDB trial, the pemetrexed/cisplatin combination showed better survival in patients with nonsquamous histology, whereas the gemcitabine/cisplatin was better in survival in patients with squamous histology16. The poor response of squamous cell carcinoma to pemetrexed has been thought to be related to the high TS level in this histology8990. Thus, TS may be a predictive marker in pemetrexed-based chemotherapy. Several previous studies have demonstrated the predictive potential of TS level in this setting919293. However, a large biomarker-stratified randomized trial failed to show difference in the response and survival of pemetrexed/cisplatin between TS-negative and TS-positive group94. In this trial, pemetrexed/cisplatin demonstrated superior RR and PFS than gemcitabine/cisplatin only in the TS-negative group, whereas the clinical outcomes of the two regimens were comparable in the TS-positive group94, which merely confirmed the previous data16. Overall, evidence is still lacking for TS-guided chemotherapy and further investigations are warranted for better understanding of the clinical implication of TS level in platinum-based chemotherapy.
Guidelines recommend chemotherapy as a subsequent treatment after TKI failure in oncogene-addictive NSCLC8910. Several previous trials comparing TKIs and chemotherapy have shown the clinical benefits of chemotherapy in oncogene-addicted lung cancer. For ALK-rearranged NSCLC, the RR was as high as 45% to first-line pemetrexed/cisplatin and 20% to pemetrexed or docetaxel as the second line treatment9596. In addition, pemetrexed showed favorable activity in some kind of driving mutations including RET and ROS1 fusions9798. As targeted therapy for those rare driving mutations is currently not readily available, chemotherapy, especially pemetrexed-based, may be an evidence-based feasible option in this setting.
Over the past decades, we have noticed huge progress in lung cancer therapeutics. Although the development in chemotherapy has not been so dramatic compared to that in molecular targeted therapy and immune-oncology, evidence clearly shows that chemotherapy is still an essential part of lung cancer treatment, regardless of stage, histology, mutational subtype, and immunologic status. The introduction of new treatment strategies, including maintenance therapy, new drug delivery systems, and combination with other classes of anti-neoplastic drugs, has demonstrated clear improvement in the survival and/or toxicity, compared to the conventional treatment. In particular, chemoimmunotherapy have provided promising data and it would be a new standard of care for nononcogene-addicted NSCLC. Overall, chemotherapy still works even in this era of precision medicine. Future investigations must be focused on the development of novel chemotherapeutic approaches with high efficacy and less toxicity, and identification of potential predictive or prognostic biomarkers to minimize the toxicity and maximize the efficacy of chemotherapy, by selecting an optimal regimen for the individual patient.
Figures and Tables
Acknowledgments
Part of this work was originally presented at the 13th Lung Cancer Symposium of the Korean Academy of Tuberculosis and Respiratory Diseases, Daegu, South Korea, April 2018. This work was supported in part by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (grant No. NRF-2017R1C1B5016828).
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis. 2016; 79:58–69.

3. Bansal P, Osman D, Gan GN, Simon GR, Boumber Y. Recent advances in targetable therapeutics in metastatic non-squamous NSCLC. Front Oncol. 2016; 6:112.

4. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–1833.

5. Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017; 35:2781–2789.

6. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–1639.

7. Kohno T, Nakaoku T, Tsuta K, Tsuchihara K, Matsumoto S, Yoh K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015; 4:156–164.
8. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27:v1–v27.

9. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017; 35:3484–3515.

10. National Comprehensive Cancer Network guidelines [Internet]. Plymouth Meeting: National Comprehensive Cancer Network;2018. cited 2018 Aug 15. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
11. Karnofsky DA. Chemotherapy of neoplastic disease: agents of clinical value. N Engl J Med. 1948; 239:299–305.
12. Rockswold GL, Ramsey HE, Buker GD. The results of treatment of lung cancer by surgery, radiation and chemotherapy at a USPHS hospital. Mil Med. 1970; 135:362–368.

13. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995; 311:899–909.
14. D'Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005; 23:2926–2936.
15. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98.

16. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008; 26:3543–3551.

17. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009; 374:1432–1440.

18. Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013; 14:777–786.

19. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018; 378:2078–2092.

20. Santabarbara G, Maione P, Rossi A, Gridelli C. Pharmacotherapeutic options for treating adverse effects of Cisplatin chemotherapy. Expert Opin Pharmacother. 2016; 17:561–570.

21. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016; 16:653–660.

22. Lima JP, dos Santos LV, Sasse EC, Sasse AD. Optimal duration of first-line chemotherapy for advanced non-small cell lung cancer: a systematic review with meta-analysis. Eur J Cancer. 2009; 45:601–607.

23. Soon YY, Stockler MR, Askie LM, Boyer MJ. Duration of chemotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009; 27:3277–3283.

24. Rossi A, Chiodini P, Sun JM, O'Brien ME, von Plessen C, Barata F, et al. Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2014; 15:1254–1262.

25. Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012; 13:247–255.

26. Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013; 31:2895–2902.

27. Belani CP, Brodowicz T, Ciuleanu TE, Krzakowski M, Yang SH, Franke F, et al. Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol. 2012; 13:292–299.

28. Paik SS, Hwang IK, Park MJ, Lee SH. Pemetrexed continuation maintenance versus conventional platinum-based doublet chemotherapy in EGFR-negative lung adenocarcinoma: retrospective analysis. Tuberc Respir Dis. 2018; 81:148–155.

29. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018; 3:7.

30. Blair HA, Deeks ED. Albumin-bound paclitaxel: a review in non-small cell lung cancer. Drugs. 2015; 75:2017–2024.

31. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012; 30:2055–2062.

32. Socinski MA, Langer CJ, Okamoto I, Hon JK, Hirsh V, Dakhil SR, et al. Safety and efficacy of weekly nab(R)-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. 2013; 24:314–321.
33. Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013; 12:329–332.

34. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367:1783–1791.

35. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018; 36:2532–2537.
36. Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015; 7:302ra136.

37. Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017; 18:42–51.

38. ClinicalTrials.gov [Internet]. Bethesda: U.S.: National Library of Medicine;2018. cited 2018 Aug 15. Available from: https://clinicaltrials.gov.
39. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355:2542–2550.

40. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009; 27:1227–1234.

41. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015; 33:2197–2204.

42. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010; 21:1804–1809.

43. Soria JC, Mauguen A, Reck M, Sandler AB, Saijo N, Johnson DH, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013; 24:20–30.

44. Wakelee HA, Dahlberg SE, Keller SM, Tester WJ, Gandara DR, Graziano SL, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017; 18:1610–1623.

45. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014; 384:665–673.

46. Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn MJ, Tiangco B, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-Lung 2): a randomized, double-blind, phase III trial. Lung Cancer. 2016; 102:65–73.

47. Fenchel K, Sellmann L, Dempke WC. Overall survival in non-small cell lung cancer-what is clinically meaningful? Transl Lung Cancer Res. 2016; 5:115–119.
48. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014; 311:1998–2006.

49. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004; 22:777–784.

50. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007; 25:1545–1552.

51. Zheng Y, Xu N, Zhou J. Intercalated chemotherapy and erlotinib: a viable first-line option for patients with advanced NSCLC. Lancet Oncol. 2013; 14:e438.

52. Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013; 2:e27025.

53. Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012; 61:2343–2356.

54. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010; 102:115–123.

55. Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology. 2017; 6:e1331807.

56. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015; 3:436–443.

57. Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013; 62:405–410.

58. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016; 17:1497–1508.

59. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004; 350:351–360.

60. Arriagada R, Dunant A, Pignon JP, Bergman B, Chabowski M, Grunenwald D, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010; 28:35–42.

61. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006; 7:719–727.

62. Douillard JY, Tribodet H, Aubert D, Shepherd FA, Rosell R, Ding K, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010; 5:220–228.

63. Burdett S, Pignon JP, Tierney J, Tribodet H, Stewart L, Le Pechoux C, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev. 2015; (3):CD011430.
64. Park SY, Lee JG, Kim J, Byun GE, Bae MK, Lee CY, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg. 2013; 8:151.

65. Robinson CG, Patel AP, Bradley JD, DeWees T, Waqar SN, Morgensztern D, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol. 2015; 33:870–876.

66. Schaake-Koning C, van den Bogaert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992; 326:524–530.

67. Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III nonsmall-cell lung cancer: a randomized study. J Clin Oncol. 1996; 14:1065–1070.

68. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011; 103:1452–1460.

69. Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016; 34:953–962.

70. Slebos RJ, Kibbelaar RE, Dalesio O, Kooistra A, Stam J, Meijer CJ, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990; 323:561–565.
71. Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, Sakurada A, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007; 25:5240–5247.
72. Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008; 26:1472–1478.

73. Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter. J Clin Oncol. 2013; 31:1112–1121.

74. Altaha R, Liang X, Yu JJ, Reed E. Excision repair cross complementing-group 1: gene expression and platinum resistance. Int J Mol Med. 2004; 14:959–970.
75. Rosell R, Lord RV, Taron M, Reguart N. DNA repair and cisplatin resistance in non-small-cell lung cancer. Lung Cancer. 2002; 38:217–227.

76. Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002; 8:2286–2291.
77. Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006; 355:983–991.

78. Simon GR, Sharma S, Cantor A, Smith P, Bepler G. ERCC1 expression is a predictor of survival in resected patients with non-small cell lung cancer. Chest. 2005; 127:978–983.

79. Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007; 25:2747–2754.

80. Lee SM, Falzon M, Blackhall F, Spicer J, Nicolson M, Chaudhuri A, et al. Randomized prospective biomarker trial of ERCC1 for comparing platinum and nonplatinum Therapy in Advanced Non-Small-Cell Lung Cancer: ERCC1 Trial (ET). J Clin Oncol. 2017; 35:402–411.

81. Toffart AC, Timsit JF, Couraud S, Merle P, Moro-Sibilot D, Perol M, et al. Immunohistochemistry evaluation of biomarker expression in non-small cell lung cancer (Pharmacogenoscan study). Lung Cancer. 2014; 83:182–188.

82. Bonanno L. Predictive models for customizing chemotherapy in advanced non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2013; 2:160–171.
83. Yeh KH, Shun CT, Chen CL, Lin JT, Lee WJ, Lee PH, et al. High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer. 1998; 82:1626–1631.

84. Harpole DH Jr, Moore MB, Herndon JE 2nd, Aloia T, D'Amico TA, Sporn T, et al. The prognostic value of molecular marker analysis in patients treated with trimodality therapy for esophageal cancer. Clin Cancer Res. 2001; 7:562–569.
85. Edler D, Kressner U, Ragnhammar P, Johnston PG, Magnusson I, Glimelius B, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res. 2000; 6:488–492.
86. Huang C, Liu D, Masuya D, Nakashima T, Kameyama K, Ishikawa S, et al. Clinical application of biological markers for treatments of resectable non-small-cell lung cancers. Br J Cancer. 2005; 92:1231–1239.

87. Shimokawa H, Uramoto H, Onitsuka T, Iwata T, Nakagawa M, Ono K, et al. TS expression predicts postoperative recurrence in adenocarcinoma of the lung. Lung Cancer. 2011; 72:360–364.

88. Bepler G, Sommers KE, Cantor A, Li X, Sharma A, Williams C, et al. Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol. 2008; 3:1112–1118.

89. Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006; 107:1589–1596.

90. Monica V, Scagliotti GV, Ceppi P, Righi L, Cambieri A, Lo Iacono M, et al. Differential thymidylate synthase expression in different variants of large-cell carcinoma of the lung. Clin Cancer Res. 2009; 15:7547–7552.

91. Hashimoto H, Ozeki Y, Sato M, Obara K, Matsutani N, Nakagishi Y, et al. Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer. 2006; 106:1595–1601.

92. Christoph DC, Asuncion BR, Hassan B, Tran C, Maltzman JD, O'Shannessy DJ, et al. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol. 2013; 8:19–30.

93. Lee SH, Noh KB, Lee JS, Lee EJ, Min KH, Hur GY, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer. 2013; 81:102–108.

94. Sun JM, Ahn JS, Jung SH, Sun J, Ha SY, Han J, et al. Pemetrexed plus cisplatin versus gemcitabine plus cisplatin according to thymidylate synthase expression in nonsquamous non-small-cell lung cancer: a biomarker-stratified randomized phase II trial. J Clin Oncol. 2015; 33:2450–2456.

95. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368:2385–2394.

96. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–2177.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download