Abstract
Objective
The absence of collateral ventilation (CV) is crucial for effective bronchoscopic lung volume reduction (BLVR) with an endobronchial valve. Here, we assessed whether CT can predict the Chartis™ results.
Materials and Methods
This study included 69 patients (mean age: 70.9 ± 6.6 years; 66 [95.7%] males) who had undergone CT to assess BLVR eligibility. The Chartis™ system (Pulmonox Inc.) was used to check CV. Experienced thoracic radiologists independently determined the completeness of fissures on volumetric CT images.
Results
The comparison between the visual and quantitative analyses revealed that 5% defect criterion showed good agreement. The Chartis™ assessment was performed for 129 lobes; 11 (19.6%) of 56 lobes with complete fissures on CT showed positive CV, while this rate was significantly higher (40 of 49 lobes, i.e., 81.6%) for lobes with incomplete fissures. The size of the fissure defect did not affect the rate of CV. Of the patients who underwent BLVR, 22 of 24 patients (91.7%) with complete fissures and three of four patients with incomplete fissures (75%) achieved target lobe volume reduction (TLVR).
Conclusion
The quantitative analysis of fissure shows that incomplete fissures increased the probability of CV on Chartis™, while the defect size did not affect the overall rates. TLVR could be achieved even in some patients with relatively large fissure defect, if they showed negative CV on Chartis™.
Chronic obstructive pulmonary disease (COPD) is a major global health problem, with a prevalence 5–25% in adults, and the fourth leading cause of death worldwide (12). COPD treatment is based on bronchodilators, which have shown beneficial effects in clinical trials (34). However, since bronchodilators act mainly on the airways rather than on the lung parenchyma, they are less effective in emphysema, which is characterized by parenchymal destruction and hyperinflation (5). Other treatments for COPD include smoking cessation, pulmonary rehabilitation, and long-term oxygen therapy. However, the positive effect of these treatments on lung function is limited.
Bronchoscopic lung volume reduction (BLVR) was developed to treat hyperinflation in advanced emphysema (678). It can improve lung function and exercise capacity in selected patients (9101112), as well as ventilation perfusion mismatch (13). The Zephyr Endobronchial Valve (Pulmonx Inc., Redwood City, CA, USA) is the most extensively studied BLVR method. For a good clinical response to BLVR with an endobronchial valve (EBV), target lobe volume reduction (TLVR) is crucial (14). To achieve TLVR, it is important to ensure that there is no collateral ventilation (CV) between the target lobe and other lobes. Two methods, computed tomography (CT) and the Chartis™ system (Pulmonx Inc.), have been used to predict the presence of CV (15). CT can predict CV with high sensitivity, but its specificity is relatively low (44%) (16). TLVR (volume reduction of the target lobe by > 50%) has been achieved in only 32.5% of patients with complete fissure on CT (14). The Chartis™ system enables the relatively precise detection of CV; in 70.6% of patients who lacked CV as defined by the Chartis™ system, TLVR has been achieved (9). For this reason, the Chartis™ system is generally recommended to be applied before EBV implantation (17).
The main limitation of Chartis™ system is that bronchoscopy is inevitable for the Chartis™ system to be used; it prolongs the total procedure time and it can increase additional risk by inducing hypoxemia in some patients with severe emphysema. In about 15% of patients, the system does not clarify the presence of CV because of blockage of the catheter by secretions, apposition of the catheter against the airway wall, or anatomical factors (1819). In addition, the Chartis™ system may be an economic burden to patients, and some patients cannot undergo BLVR even after the Chartis™ assessment if definite CV is present. Since CT is a usual evaluation process for BLVR, substituting the Chartis™ with a CT assessment at least in a number of patients can shorten the procedure time.
Previous studies showed that CT evaluation for incomplete fissures shows good correlation with the predictions of the Chartis™ system (162021). However, these studies used various definitions of incomplete fissures and did not perform quantitative analyses. In this study, we quantitatively assessed measurements of fissures and fissure defects. We assessed how well the extent of fissure integrity can be evaluated on CT, and analyzed whether the results can be applied to predict the results of the Chartis™ system or TLVR outcomes after BLVR.
Of all patients with advanced emphysema referred to our tertiary care referral hospital between July 2012 and December 2013 who did not respond to the usual COPD treatment and were considered for BLVR, we retrospectively reviewed all patients who underwent CT to evaluate their eligibility for BLVR. Their fissure integrity evaluation results from CT, their Chartis® System assessment, and the results of EBV insertions were assessed. BLVR was recommended if the following criteria, suggested by previous studies were met (622): age of 40 years or older, diagnosis of heterogeneous emphysema, forced expiratory volume in one second (FEV1) of 15% to 50% of the predicted value, total lung capacity of more than 100% of the predicted value, and residual volume of more than 150% of the predicted value. The exclusion criteria included a diffusing capacity corrected for alveolar volume of less than 20% of the predicted value, the presence of giant bullae (> 3 cm in diameter) on the ipsilateral side of the target lobe, excessive sputum production, severe pulmonary hypertension, and an unstable cardiac condition. This study was approved by the Institutional Review Board of institution.
Before the BLVR procedure, the patients received the maximal medical therapy for at least 3 months, including pharmacological and non-pharmacological treatments, as presented in the Global Initiative for Chronic Obstructive Lung Disease guidelines (1). After providing informed consent, patients underwent pulmonary function tests, the 6-minute walking distance test, and chest CT. A Zephyr® Endobronchial Valve was placed via a flexible bronchoscope under conscious sedation, as described previously (61022). Before placing the EBVs, the Chartis™ system was used to test for CV of several candidate lobes (9). CV was deemed negative when the air flow declined gradually over time after occlusion of the target lobe airway with a balloon catheter and inspiratory pressure became maximized. However, if persistent airflow was observed after occlusion for more than 5 minutes, CV was defined as positive (2324), and we had not inserted EBV in these cases (12). One experienced bronchoscopist (bronchoscopy experience of 12 years, EBV insertion for more than 30 cases) performed the whole procedure. To ensure the Chartis™ results, the other parts of target lobe (e.g., bronchus intermedius for right upper lobe [RUL]) were also measured, if necessary.
Patients underwent chest CT using the SOMATOM Defintion Flash CT system (Siemens Healthineers, Forchheim, Germany) before BLVR. We used 1-mm thickness axial/sagittal CT images of the entire thorax with full inspiration to evaluate fissure completeness (512 × 512 matrix; 64 × 0.6 mm collimation; 89/210 effective mAs at Sn140/80 kV; 0.55 pitch; 0.28 second rotation time). If BLVR was performed, patients underwent chest CTs following the same protocol.
Four experienced thoracic radiologists independently determined the fissure completeness. To match the results to the Chartis™ test results, a right major fissure was subdivided into upper and lower portions at the contact point of the minor fissure, and four fissures (upper portion of right major fissure, lower portion of right major fissure, right minor fissure, and left major fissure) were entered into the analysis. Fissure completeness was defined using two kinds of criteria: 1) no fissure discontinuity, if there was a disagreement, completeness was determined by a third experienced radiologist or 2) < 10% fissure discontinuity using both axial and sagittal CT images.
In cases of incomplete fissures, two readers measured the maximal defect sizes on both axial and sagittal planes and calculated an approximate defect area (axial defect size × sagittal defect size × 3.14/4, mm2). The final defect size estimation was calculated as the mean value of the results. The lobes were semi-automatically segmented by a radiology technician under the supervision of a radiologist. After segmentation of each lobe, the fissure areas were measured based on the borders of each lobe (Fig. 1). We calculated the percentage area of the fissure defect on each fissure. Interpretation of fissure completeness by readers was determined using different criteria (5%, 10%, and 15%).
Using the results of the lobe segmentation before and after BLVR, each lobe volume was calculated. Target volume reduction was defined as 1) ≥ 350 mL or 2) 50% of the initial volume of the target lobes.
To calculate interobserver variability, the agreement between decisions and measurements by two readers was assessed using kappa statistics and an interclass correlation coefficient (ICC) according to the following variables: axial and sagittal defect size (mm), axial defect area (mm2), and fissure completeness, for each of the two readers. For comparison between visual completeness using the 10% defect criterion and quantitative results of fissure completeness, kappa statistics were used. The fissure completeness of each patient was compared with the presence of CV, assessed by the Chartis™ test in 71 lobes. P value of less than 0.05 was considered statistically significant.
A total of 69 patients with emphysema met the eligibility criteria and underwent examinations including CT scans to determine if they were appropriate candidates for BLVR (group 1). After excluding 19 patients who were judged as inappropriate for BLVR, 50 patients underwent bronchoscopy for BLVR accompanied by the Chartis™ procedure (group 2). Of these patients, 15 were excluded from EBV insertion. Finally, 35 patients underwent BLVR with EBV insertion (group 3) (Fig. 2).
The demographic characteristics of the three groups were similar; the mean age was 71 years, more than 90% of patients were male, and the mean body mass index was below 20 kg/m2 in all groups. All patients had a history of smoking. Most of them were using long-acting bronchodilators and inhaled corticosteroids. All had poor pulmonary function, with an FEV1 of 0.79 ± 0.38 L in group 1, 0.76 ± 0.28 L in group 2, and 0.74 ± 0.29 L in group 3. Mean residual volumes were above 190% of the predicted value in all groups (Table 1).
A total of 276 fissures in 69 patients were evaluated. Mean fissure areas were 94.9 ± 24.4 cm2 in right minor fissure, 183.6 ± 59.9 cm2 in right major fissure and 180.2 ± 48.7 cm2 in left major fissure. Fissure completeness using 0% defect criterion showed good agreement between the two readers (kappa value of 0.786) (Table 2). Fissure completeness assessment of each reader showed very good agreement with final completeness decision (kappa values of 0.880 and 0.910). Fissure completeness using 10% defect criterion also showed good agreement between readers (kappa value of 0.759) (Table 2). In total, 118 fissures (24 right upper major fissures, 24 right lower major fissures, 32 right minor fissures and 38 left major fissures) in 55 patients (42.8%) were incomplete. Measured axial/sagittal defect sizes and areas were 17.4 ± 25.3 mm (axial), 17.6 ± 26.0 mm (sagittal), 728.3 ± 1374.9 mm2 (area), and 16.1 ± 23.2 mm, 17.5 ± 26.0 mm, 662.9 ± 1229.8 mm2, respectively. As for the reliability of defect measurements, the ICC values for axial/sagittal sizes and areas of defects were 83.9%, 88.0%, and 80.9%.
According to our quantitative analysis, 48 fissures among the 118 incomplete fissures, assessed by the Chartis™ test, had defects of 10% or less. In the visual analysis, reader 1 and reader 2 judged nine and 24 fissures as showing defects of less than 10%. The comparison between the visual and quantitative analyses revealed that a quantitative analysis with a 5% defect criterion showed good and very good agreement with the results of the two readers (kappa values of 0.726 and 0.821) (Table 3).
The Chartis™ assessment was performed for 138 lobes in group 2, including 30 RUL, 25 right middle lobe (RML), 29 right lower lobe (RLL), four right bronchus intermedius (RBI), 18 left upper lobe (LUL), and 32 left lower lobe (LLL). Evaluation of 105 (76.1%) of these lobes with the Chartis™ system gave conclusive results, while 33 (23.9%) assessments yielded inconclusive results, including 13 cases of abrupt flow cessation and 20 cases of technical failures, such as secretion and apposition of the catheter against the airway wall (19).
Among the 105 lobes with conclusive Chartis™ results, 56 showed completeness without any defect on CT. For 11 of these (19.6%), the Chartis™ system indicated positive CV, while 40 of 49 lobes (81.6%) with fissure defects showed positive CV on the Chartis™ system; this rate was significantly higher than that found for complete fissures (p < 0.001). In the lobes with fissure defects, positive CV rates were between 75.0% and 87.5%. Rates of positive CV did not significantly differ with the size or proportion of the fissure defect (Fig. 3).
Thirty-five patients underwent BLVR (group 3); all showed negative CV on the Chartis™ system, except for three with inconclusive results. The treated lobes included 13 RUL, 4 RLL, 2 RUL and RML, 1 LUL, and 15 LLL. Twenty-four patients underwent a follow-up CT assessment 92 ± 10 days after BLVR; 22 (91.7%) showed TLVR > 350 mL, and 16 (66.7%) showed TLVR of > 50% of the initial volume (Fig. 4). The difference in TLVR rates (> 50% of reduction) was not noted according to the lobes treated (9/14 in right lung, 7/10 in left lung, p = 0.77). Although four patients showed incomplete fissures on CT, in three (75%) of these, TLVR > 350 mL was achieved, and in two (50%), TLVR of > 50%. The rate of TLVR achievement was higher in patients with complete fissures, but the difference was not statistically significant (p = 0.18 for TLVR > 350 mL, p = 0.48 for TLVR > 50%) (Table 4). Four patients had pneumothorax after procedure, and all of them had complete fissure without defect area (0%) around the target lobes.
This study shows that the measurement of fissure defect is well matched between readers. The presence of fissure defect on CT affects the results of the Chartis™ system significantly. We found a rate of CV of less than 20% in cases of complete fissures, but this number increased to 80% in cases of incomplete fissures, even small ones, suggesting that the size of the defect does not impact the results. The fissure completeness on CT could not exclude the necessity of Chartis™ completely, because some patients still had TLVR despite large fissure defect.
With negative CV, we can expect higher response rate (1525). We also do not have enough evidence to insert EBV, for patients with CV present (21). Whether CT can predict CV is the important issue, recent review recommended that CT-fissure integrity ≥ 95%, valves could be directly implanted without using Chartis™ (26). The main basis is that CT with this criterion of fissure integrity ≥ 90% or 95% has comparable accuracy to predict TLVR with Chartis™ (212427). However, patients with complete fissure still did not have enough chance of TLVR of 350 mL (57.1% (27) and 66.7% (21)) if CV present. Complete fissure on CT also could not exclude CV on Chartis™ (2124). Therefore, the effort to predict CV is still valuable.
Interestingly, the results of Chartis™ were different between 0% and 0.1–5% fissure defect, indicating 19.6% and 87.5% of positive CV in our study. Our data also indicate that the defect size does not impact the presence of CV, as even defects of less than 5 cm2 or 5% led to 80% of CV. This may be some clue to why relatively large portion of patients with 95% of fissure integrity still have positive CV and do not have TLVR. This indicated that fissure integrity should be stricter and more complete to exclude CV. The patients group with fissure integrity of ≥ 90% or ≥ 95% in previous studies might include relatively heterogeneous group of 100% and less than 100%. A recent study also corroborated the use of this criterion by showing higher response rates in fissure defects of less than 10% (27). However, the non-responder rate also reached 28% in these cases with “complete fissures,” suggesting that minimal fissure defects still contributed to CV. In another study, odds ratio of TLVR increased by 1.47 with every 5% increase of fissure integrity (27). This study also showed that notable increase of TLVR rate with fissure integrity ≥ 90%. This notable increase may come from the inclusion of patients with fissure integrity of 100% in this group.
One of the goals of this study was to determine the fissure defect size that indicates definite CV. A previous study showed that the negative predictive value for lobar atelectasis was 100% in patients with fissure defect above 25% (20). In our study, fissure defect above 20% was noted in 8 lobes. Among them, Chartis™ showed negative CV in two lobes (22.7% and 22.8% of fissure defect, respectively) and one of them achieved TLVR after EBV insertion (Fig. 4). Meanwhile, all of four lobes with fissure defect above 25% (27.2%, 27.4%, 30.3%, and 31.9%) showed positive CV. Therefore, recent suggestion about fissure defect of 25% is still applied to our finding that even Chartis™ may be futile in these cases (26).
TLVR was achieved in 91.6% of patients (≥ 350 mL). Among patients with fissure integrity of 100%, this rate increased to 95%. This response rate was higher than those of previous studies without exclusion of positive CV (14) and similar with those applying CV exclusion (101228). We inserted EBVs only when the Chartis™ system indicated definite negative CV with a few exception (19). In real-world practice of our country, EBV insertion was not easy in the case with positive CV, because the patients usually wanted guaranteed treatment response due to economic burden for insufficient insurance coverage. In two patients, TLVR of < 350 mL could not be achieved, and in six patients, < 50% of the initial volume could not be reached. One of these patients did not even show a fissure defect around the target lobe. In contrast, TLVR was achieved in some cases with definite and large fissure defects.
The presence and measurements of fissure defects showed very good agreement. However, visual assessment of defect area percentage showed slightly lower agreements, maybe due to the subjective evaluation. Regarding the consistency of the decision, 0% defect criterion seems more suitable than 10% defect criterion. Furthermore, quantitative analysis revealed a larger number of incomplete fissures than visual analysis. This could be due to subjective differences in reader evaluations or to limitations of quantification method. Recently, automated or semiautomatic evaluation of fissure completeness is possible using analysis software (20). Automated evaluation of fissure completeness can reduce the subjective differences and analysis time. Further studies are needed to evaluate whether quantitative methods are indeed more consistent than visual analysis.
This study has several limitations. First, BLVR was not performed in patients with positive CV, and we could therefore not evaluate the benefit of TLVR and the positive predictive value of the Chartis™ system. However, since the absence of CV is an important indicator of the clinical response, BLVR with an EBV can currently not be recommended for patients with CV (1229). Second, it is not easy to interpret TLVR results after EBV implantation for patients with definite fissure defect on CT. The size of the pores of Kohn is 1–12 µm (30). and that of the canals of Lambert is about 200 µm (31). Since CT cannot allow calculation of sizes at this resolution, it has a clear limitation regarding the prediction of the absence of CV. Meanwhile, there is no clear answer as to how TLVR could be achieved in some patients with definite incomplete fissures.
In conclusion, quantitative analysis of incomplete fissures is feasible. The presence of incomplete fissures indicates higher CV rates, even if the defect is small. Meanwhile, the size of the fissure defect does not lead to differences in the rate of CV, and TLVR may be achieved even in patients with relatively large incomplete areas if CV is negative, but the possibility is scarce. These findings have direct clinical implications and offer important guidelines for the selection of target patients for BLVR.
Acknowledgments
The authors thank Taekjin Jang, BS and Heejun Park, BS, for their technical assistance.
Notes
References
1. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365. PMID: 22878278.
2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006; 3:e442. PMID: 17132052.

3. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789. PMID: 17314337.

4. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554. PMID: 18836213.

5. Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010; 104:542–549. PMID: 19926461.

6. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010; 363:1233–1244. PMID: 20860505.

7. Snell GI, Holsworth L, Borrill ZL, Thomson KR, Kalff V, Smith JA, et al. The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest. 2003; 124:1073–1080. PMID: 12970040.
8. Wan IY, Toma TP, Geddes DM, Snell G, Williams T, Venuta F, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006; 129:518–526. PMID: 16537847.
9. Herth FJ, Eberhardt R, Gompelmann D, Ficker JH, Wagner M, Ek L, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J. 2013; 41:302–308. PMID: 22556025.

10. Herth FJ, Noppen M, Valipour A, Leroy S, Vergnon JM, Ficker JH, et al. International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012; 39:1334–1342. PMID: 22282552.

11. Park TS, Hong Y, Lee JS, Oh SY, Lee SM, Kim N, et al. Bronchoscopic lung volume reduction by endobronchial valve in advanced emphysema: the first Asian report. Int J Chron Obstruct Pulmon Dis. 2015; 10:1501–1511. PMID: 26251590.
12. Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015; 373:2325–2335. PMID: 26650153.

13. Lee SW, Lee SM, Shin SY, Park TS, Oh SY, Kim N, et al. Improvement in ventilation-perfusion mismatch after bronchoscopic lung volume reduction: quantitative image analysis. Radiology. 2017; 285:250–260. PMID: 28510483.

14. Valipour A, Herth FJ, Burghuber OC, Criner G, Vergnon JM, Goldin J, et al. VENT Study Group. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J. 2014; 43:387–396. PMID: 23845721.

15. Shah PL, Herth FJ. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax. 2014; 69:280–286. PMID: 24008689.

16. Reymond E, Jankowski A, Pison C, Bosson JL, Prieur M, Aniwidyaningsih W, et al. Prediction of lobar collateral ventilation in 25 patients with severe emphysema by fissure analysis with CT. AJR Am J Roentgenol. 2013; 201:W571–W575. PMID: 24059394.

17. Slebos DJ, Shah PL, Herth FJ, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017; 93:138–150. PMID: 27992862.

18. Gompelmann D, Eberhardt R, Herth FJ. Collateral ventilation. Respiration. 2013; 85:515–520. PMID: 23485627.

19. Shah PL, Herth FJ. Dynamic expiratory airway collapse and evaluation of collateral ventilation with Chartis. Thorax. 2014; 69:290–291. PMID: 24355826.

20. de Oliveira HG, de Oliveira SM, Rambo RR, de Macedo Neto AV. Fissure integrity and volume reduction in emphysema: a retrospective study. Respiration. 2016; 91:471–479. PMID: 27241515.

21. Gompelmann D, Eberhardt R, Slebos DJ, Brown MS, Abtin F, Kim HJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology. 2014; 19:524–530. PMID: 24612306.

22. Venuta F, Anile M, Diso D, Carillo C, De Giacomo T, D'Andrilli A, et al. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J. 2012; 39:1084–1089. PMID: 22005916.

23. Gompelmann D, Eberhardt R, Michaud G, Ernst A, Herth FJ. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration. 2010; 80:419–425. PMID: 20664194.

24. Gesierich W, Samitas K, Reichenberger F, Behr J. Collapse phenomenon during Chartis collateral ventilation assessment. Eur Respir J. 2016; 47:1657–1667. PMID: 27076587.

25. Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015; 386:1066–1073. PMID: 26116485.

26. Fiorelli A, Santini M, Shah P. When can computed tomography-fissure analysis replace Chartis collateral ventilation assessment in the prediction of patients with emphysema who might benefit from endobronchial valve therapy? Interact Cardiovasc Thorac Surg. 2018; 26:313–318. PMID: 29049667.

27. Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med. 2015; 191:767–774. PMID: 25635349.

28. Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. IMPACT Study Team. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med. 2016; 194:1073–1082. PMID: 27580428.

29. Davey C, Zoumot Z, Jordan S, Carr DH, Polkey MI, Shah PL, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi trial): study design and rationale. Thorax. 2015; 70:288–290. PMID: 24664535.

30. Desplechain C, Foliguet B, Barrat E, Grignon G, Touati F. [The pores of Kohn in pulmonary alveoli]. Bull Eur Physiopathol Respir. 1983; 19:59–68. PMID: 6850150.
31. Cotes JE, Chinn DJ, Miller MR. Lung function: physiology, measurement and application in medicine. Malden, MA: Blackwell Publishing Ltd;2006. p. 27.
Fig. 1
Semi-automatic lobe segmentation and fissure assessment.
A. Axial CT image with semi-automatic lobe segmentation result of left lung (green: left upper lobe, yellow: left lower lobe). B, C. Three-dimensional volume rendering image of left lung (green: left upper lobe, yellow: left lower lobe, red: left major fissure).
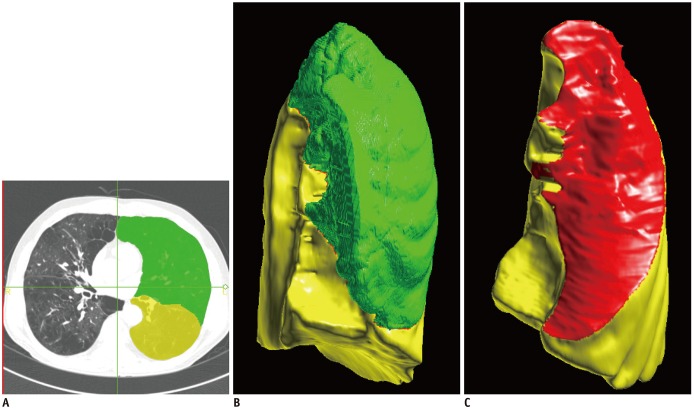
Fig. 2
Flow chart of study patients.
BLVR = bronchoscopic lung volume reduction, CV = collateral ventilation, EBV = endobronchial valve
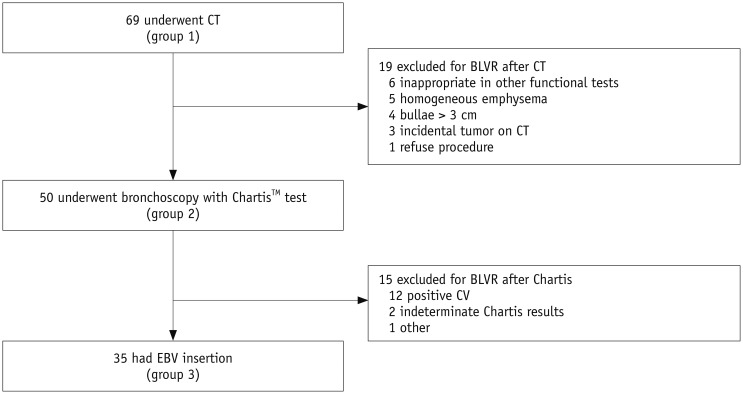
Fig. 3
Results of Chartis™ system (Pulmonx Inc.) according to fissure completeness.
In lobes without fissural defects, Chartis™ showed positive CV in 19.6% (11/56). Meanwhile, ratio increased to 79.5% (35/44) in lobes with any fissure defect, significantly higher than ratio for lobes with complete fissure (p < 0.001). In lobes with fissure defects, rates of positive CV did not depend on size or proportion of fissure defect.
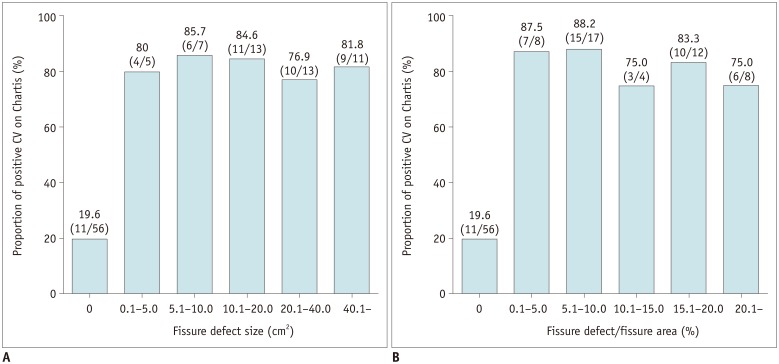
Fig. 4
Some patients showed discrepant results regarding fissure completeness and TLVR after EBV insertion.
A. Two experienced radiologists agreed that 77-year-old male had complete fissure around target lobe (RUL). B. However, TLVR was not achieved in this patient 3 months after BLVR; volume of RUL changed from 1146 mL to 808 mL (29.5% reduction). C. 58-year-old male had incomplete fissure around target lobe (RUL), with size of 43.4 cm2 (7.5 × 7.4 cm, 22.7% of total fissure area). D. Despite this incompleteness, TLVR was achieved in this patient; volume of RUL was reduced from 1012 mL to 207 mL (79.5% reduction). Chartis™ system showed negative CV in target lobes of both patients. Circles mean RUL. RUL = right upper lobe, TLVR = target lobe volume reduction
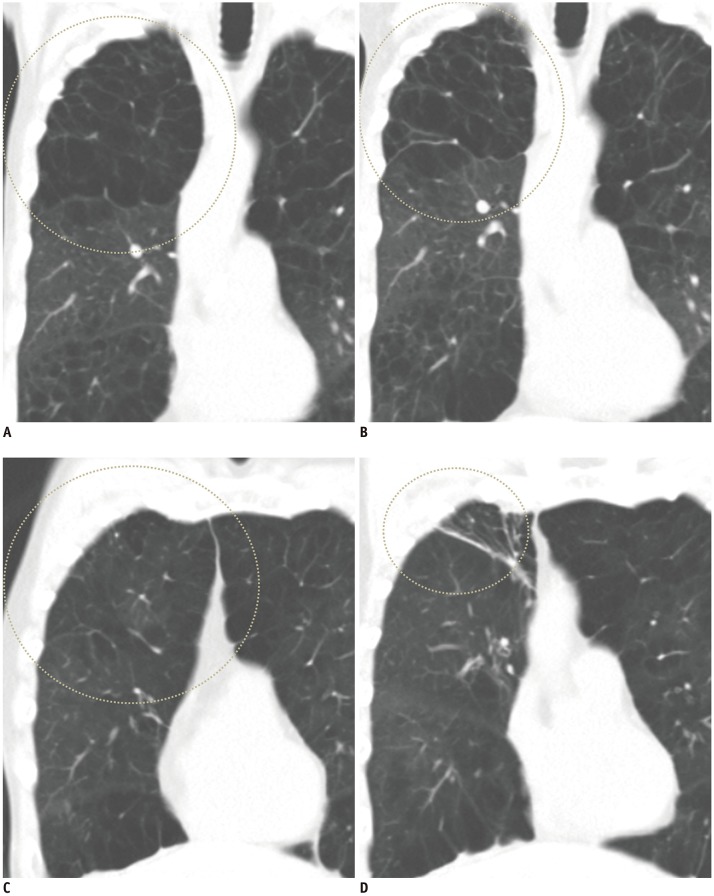
Table 1
Baseline Characteristics
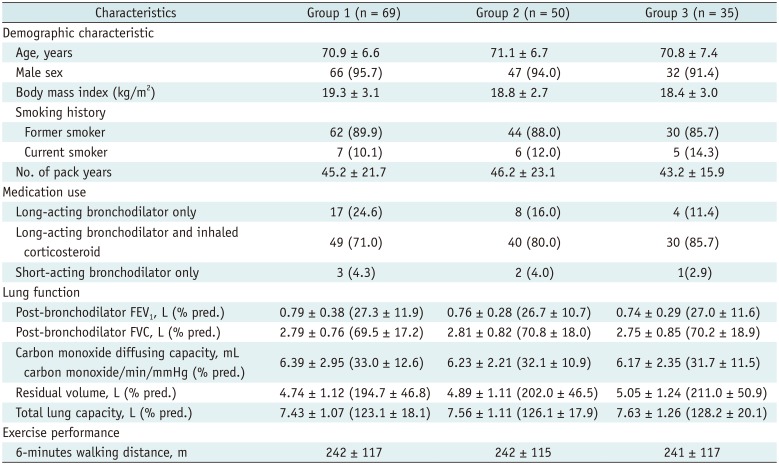
Table 2
Kappa Statistics between Two Readers with Criteria of 0% and 10%
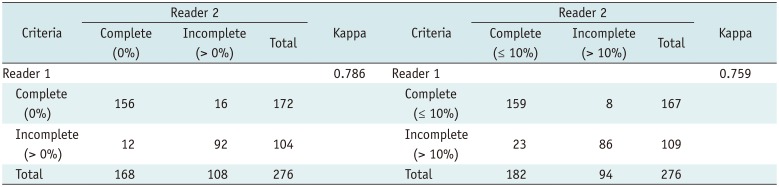
Table 3
Kappa Statistics between Visual Analysis and Quantitative Analysis
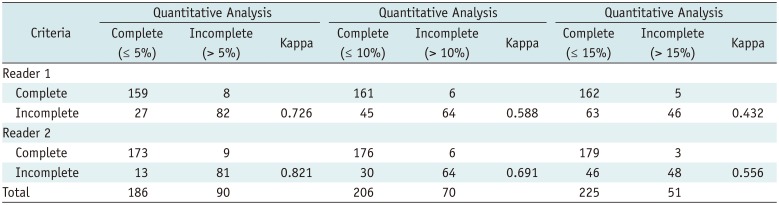
Table 4
TLVR Achievement according to Fissure Completeness on CT




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download