1. Zuckerman JD, Rokito A. Frozen shoulder: a consensus definition. J Shoulder Elbow Surg. 2011; 20:322–325. PMID:
21051244.

2. Hsu JE, Anakwenze OA, Warrender WJ, Abboud JA. Current review of adhesive capsulitis. J Shoulder Elbow Surg. 2011; 20:502–514. PMID:
21167743.

3. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: review of imaging and treatment. J Med Imaging Radiat Oncol. 2013; 57:633–643. PMID:
24283550.

4. Brue S, Valentin A, Forssblad M, Werner S, Mikkelsen C, Cerulli G. Idiopathic adhesive capsulitis of the shoulder: a review. Knee Surg Sports Traumatol Arthrosc. 2007; 15:1048–1054. PMID:
17333122.

5. Zappia M, Di Pietto F, Aliprandi A, Pozza S, De Petro P, Muda A, et al. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging. 2016; 7:365–371. PMID:
27107871.

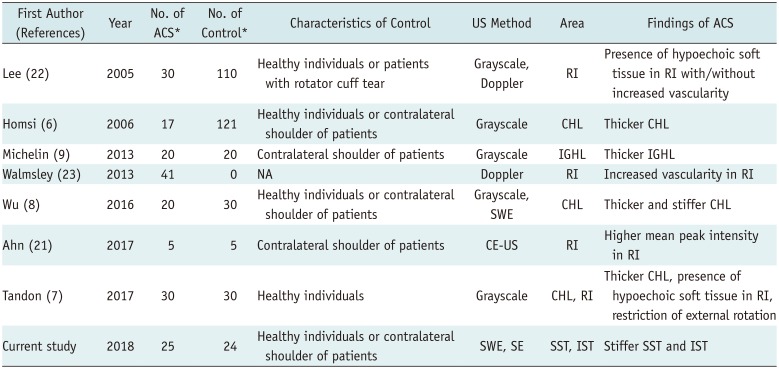
6. Homsi C, Bordalo-Rodrigues M, da Silva JJ, Stump XM. Ultrasound in adhesive capsulitis of the shoulder: is assessment of the coracohumeral ligament a valuable diagnostic tool? Skeletal Radiol. 2006; 35:673–678. PMID:
16724200.

7. Tandon A, Dewan S, Bhatt S, Jain AK, Kumari R. Sonography in diagnosis of adhesive capsulitis of the shoulder: a case-control study. J Ultrasound. 2017; 20:227–236. PMID:
28900523.

8. Wu CH, Chen WS, Wang TG. Elasticity of the coracohumeral ligament in patients with adhesive capsulitis of the shoulder. Radiology. 2016; 278:458–464. PMID:
26323030.

9. Michelin P, Delarue Y, Duparc F, Dacher JN. Thickening of the inferior glenohumeral capsule: an ultrasound sign for shoulder capsular contracture. Eur Radiol. 2013; 23:2802–2806. PMID:
23652851.

10. Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol. 2010; 14:323–333. PMID:
20539957.

11. Ooi CC, Malliaras P, Schneider ME, Connell DA. “Soft, hard, or just right?” Applications and limitations of axial-strain sonoelastography and shear-wave elastography in the assessment of tendon injuries. Skeletal Radiol. 2014; 43:1–12. PMID:
23925561.

12. Pedersen M, Fredberg U, Langberg H. Sonoelastography as a diagnostic tool in the assessment of musculoskeletal alterations: a systematic review. Ultraschall Med. 2012; 33:441–446. PMID:
22744444.

13. DePalma AF. The classic. Loss of scapulohumeral motion (frozen shoulder). Ann Surg. 1952;135:193–204. Clin Orthop Relat Res. 2008; 466:552–560. PMID:
18264843.
14. Crass JR, Craig EV, Feinberg SB. Clinical significance of sonographic findings in the abnormal but intact rotator cuff: a preliminary report. J Clin Ultrasound. 1988; 16:625–634. PMID:
3142923.

15. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005; 47:458–472. PMID:
16161804.

16. Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003; 227:617–622. PMID:
12773666.

17. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID:
843571.

18. Tuite MJ, Small KM. Imaging evaluation of nonacute shoulder pain. AJR Am J Roentgenol. 2017; 209:525–533. PMID:
28537759.

19. Schmalzl J, Fenwick A, Boehm D, Gilbert F. The application of ultrasound elastography in the shoulder. J Shoulder Elbow Surg. 2017; 26:2236–2246. PMID:
29031414.

20. Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017; 37:855–870. PMID:
28493799.

21. Ahn KS, Kang CH, Jeong WK. Contrast-enhanced ultrasonography in patients with adhesive capsulitis: preliminary experience. Iran J Radiol. 2017; 14:e33069.

22. Lee JC, Sykes C, Saifuddin A, Connell D. Adhesive capsulitis: sonographic changes in the rotator cuff interval with arthroscopic correlation. Skeletal Radiol. 2005; 34:522–527. PMID:
15999280.

23. Walmsley S, Osmotherly PG, Walker CJ, Rivett DA. Power Doppler ultrasonography in the early diagnosis of primary/idiopathic adhesive capsulitis: an exploratory study. J Manipulative Physiol Ther. 2013; 36:428–435. PMID:
23830711.

24. Li JQ, Tang KL, Wang J, Li QY, Xu HT, Yang HF, et al. MRI findings for frozen shoulder evaluation: is the thickness of the coracohumeral ligament a valuable diagnostic tool? PLoS One. 2011; 6:e28704. PMID:
22163326.

25. Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol. 2004; 14(Suppl 8):P11–P15. PMID:
15700328.
26. Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol. 2011; 21:604–615. PMID:
20927527.

27. Yasuda K, Hayashi K. Changes in biomechanical properties of tendons and ligaments from joint disuse. Osteoarthritis Cartilage. 1999; 7:122–129. PMID:
10367020.

28. Herbert RD, Crosbie J. Rest length and compliance of non-immobilised and immobilised rabbit soleus muscle and tendon. Eur J Appl Physiol Occup Physiol. 1997; 76:472–479. PMID:
9367288.

29. Ahn KS, Kang CH, Kim Y, Jeong WK. Diagnosis of adhesive capsulitis: comparison of contrast-enhanced MRI with noncontrast-enhanced MRI. Clin Imaging. 2015; 39:1061–1067. PMID:
26362354.

30. Connell D, Padmanabhan R, Buchbinder R. Adhesive capsulitis: role of MR imaging in differential diagnosis. Eur Radiol. 2002; 12:2100–2106. PMID:
12136330.

31. Gokalp G, Algin O, Yildirim N, Yazici Z. Adhesive capsulitis: contrast-enhanced shoulder MRI findings. J Med Imaging Radiat Oncol. 2011; 55:119–125. PMID:
21501399.

32. Gondim Teixeira PA, Balaj C, Chanson A, Lecocq S, Louis M, Blum A. Adhesive capsulitis of the shoulder: value of inferior glenohumeral ligament signal changes on T2-weighted fat-saturated images. AJR Am J Roentgenol. 2012; 198:W589–W596. PMID:
22623575.
33. Lee SU, Joo SY, Kim SK, Lee SH, Park SR, Jeong C. Real-time sonoelastography in the diagnosis of rotator cuff tendinopathy. J Shoulder Elbow Surg. 2016; 25:723–729. PMID:
26794853.

34. Sasanuma H, Sugimoto H, Fujita A, Kanaya Y, Iijima Y, Saito T, et al. Characteristics of dynamic magnetic resonance imaging of idiopathic severe frozen shoulder. J Shoulder Elbow Surg. 2017; 26:e52–e57. PMID:
27539943.

35. Song KD, Kwon JW, Yoon YC, Choi SH. Indirect MR arthrographic findings of adhesive capsulitis. AJR Am J Roentgenol. 2011; 197:W1105–W1109. PMID:
22109326.

36. Yoon JP, Chung SW, Lee BJ, Kim HS, Yi JH, Lee HJ, et al. Correlations of magnetic resonance imaging findings with clinical symptom severity and prognosis of frozen shoulder. Knee Surg Sports Traumatol Arthrosc. 2017; 25:3242–3250. PMID:
26611904.

37. Hou SW, Merkle AN, Babb JS, McCabe R, Gyftopoulos S, Adler RS. Shear wave ultrasound elastographic evaluation of the rotator cuff tendon. J Ultrasound Med. 2017; 36:95–106. PMID:
27914201.

38. Kocyigit F, Kuyucu E, Kocyigit A, Herek DT, Savkin R, Aslan UB. Investigation of biomechanical characteristics of intact supraspinatus tendons in subacromial impingement syndrome: a cross-sectional study with real-time sonoelastography. Am J Phys Med Rehabil. 2016; 95:588–596. PMID:
26829089.
39. Tudisco C, Bisicchia S, Stefanini M, Antonicoli M, Masala S, Simonetti G. Tendon quality in small unilateral supraspinatus tendon tears. Real-time sonoelastography correlates with clinical findings. Knee Surg Sports Traumatol Arthrosc. 2015; 23:393–398. PMID:
23771348.

40. Botar-Jid C, Damian L, Dudea SM, Vasilescu D, Rednic S, Badea R. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason. 2010; 12:120–126. PMID:
21173939.
41. Baumer TG, Dischler J, Davis L, Labyed Y, Siegal DS, van Holsbeeck M, et al. Effects of age and pathology on shear wave speed of the human rotator cuff. J Orthop Res. 2018; 36:282–288. PMID:
28657192.

42. Hsiao MY, Chen YC, Lin CY, Chen WS, Wang TG. Reduced patellar tendon elasticity with aging: in vivo assessment by shear wave elastography. Ultrasound Med Biol. 2015; 41:2899–2905. PMID:
26304500.
43. Arda K, Ciledag N, Aktas E, Aribas BK, Köse K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011; 197:532–536. PMID:
21862792.

44. Slane LC, Martin J, DeWall R, Thelen D, Lee K. Quantitative ultrasound mapping of regional variations in shear wave speeds of the aging Achilles tendon. Eur Radiol. 2017; 27:474–482. PMID:
27236815.

45. Aubry S, Risson JR, Kastler A, Barbier-Brion B, Siliman G, Runge M, et al. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skeletal Radiol. 2013; 42:1143–1150. PMID:
23708047.

46. Peltz CD, Haladik JA, Divine G, Siegal D, van Holsbeeck M, Bey MJ. ShearWave elastography: repeatability for measurement of tendon stiffness. Skeletal Radiol. 2013; 42:1151–1156. PMID:
23640400.

47. Ewertsen C, Carlsen JF, Christiansen IR, Jensen JA, Nielsen MB. Evaluation of healthy muscle tissue by strain and shear wave elastography - Dependency on depth and ROI position in relation to underlying bone. Ultrasonics. 2016; 71:127–133. PMID:
27336792.

48. Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol. 2009; 64:1196–1202. PMID:
19913130.

49. Park S, Lee DH, Yoon SH, Lee HY, Kwack KS. Evaluation of adhesive capsulitis of the shoulder with fat-suppressed T2-weighted MRI: association between clinical features and MRI findings. AJR Am J Roentgenol. 2016; 207:135–141. PMID:
27070051.

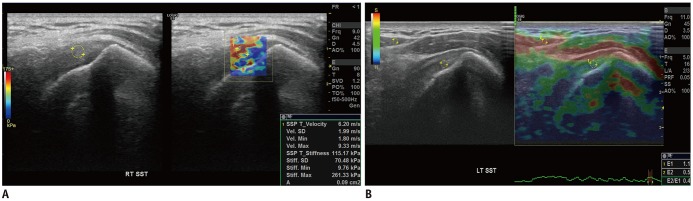
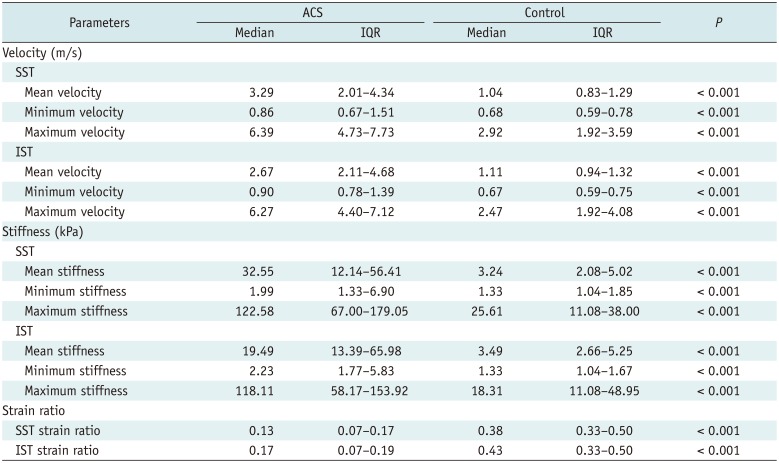
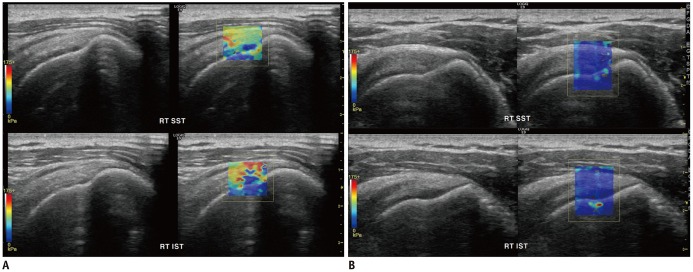
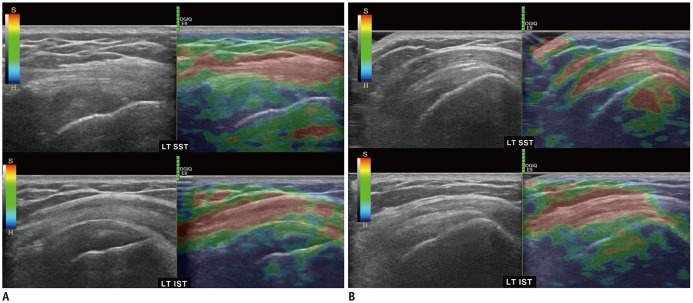




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download