1. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–926. PMID:
18436948.

2. Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008; 336:995–998. PMID:
18456631.

3. Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008; 336:1049–1051. PMID:
18467413.

4. Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008; 336:1106–1110. PMID:
18483053.

5. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010; 182:1045–1052. PMID:
20513780.

6. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010; 182:E472–E478. PMID:
20513779.

7. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380:2095–2128. PMID:
23245604.
8. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385:117–171. PMID:
25530442.
9. Lee KS, Chang HS, Lee SM, Park EC. Economic burden of cancer in Korea during 2000–2010. Cancer Res Treat. 2015; 47:387–398. PMID:
25672582.

10. Kim BH, Lim YS, Kim EY, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018; 33:475–483. PMID:
28612951.

11. Korea Central Cancer Registry. Annual report of Korean Central Cancer Registry 2015. Goyang: National Cancer Center;2017.
12. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018; 68:526–549. PMID:
28989095.

13. Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009; 101:1348–1355. PMID:
19759364.

14. World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017-recommendations. Vaccine. 2019; 37:223–225. PMID:
28743487.
15. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012; 57:167–185. PMID:
22436845.
16. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016; 10:1–98.

17. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016; 65:1140–1147. PMID:
27469901.
18. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004; 126:460–468. PMID:
14762783.

19. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013; 144:323–332. PMID:
23063971.

20. Choi J, Roberts LR. Statins and metformin for chemoprevention of hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2016; 8:48–52. PMID:
31041062.

21. Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013; 62:606–615. PMID:
22773548.

22. Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012; 104:1808–1814. PMID:
23197492.

23. Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017; 66:1556–1569. PMID:
28617992.

24. Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014; 11:45–54. PMID:
23938452.

25. Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013; 11:1413–1421.e1. PMID:
23660416.

26. Inoue M, Yoshimi I, Sobue T, Tsugane S. JPHC Study Group. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst. 2005; 97:293–300. PMID:
15713964.

27. Gelatti U, Covolo L, Franceschini M, et al. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005; 42:528–534. PMID:
15868652.

28. Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015; 148:118–125. PMID:
25305507.

29. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016; 22:18–75. PMID:
27044762.
30. Korean Association for the Study of the Liver. KASL clinical practice guidelines: management of hepatitis C. Clin Mol Hepatol. 2016; 22:76–139. PMID:
27044763.
31. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004; 351:1521–1531. PMID:
15470215.

32. Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013; 58:1537–1547. PMID:
23389810.

33. Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015; 121:3631–3638. PMID:
26177866.

34. Colombo M, Iavarone M. Role of antiviral treatment for HCC prevention. Best Pract Res Clin Gastroenterol. 2014; 28:771–781. PMID:
25260307.

35. Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014; 147:152–161. PMID:
24583062.

36. Thiele M, Gluud LL, Dahl EK, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma and mortality in chronic hepatitis B: systematic review and meta-analysis. BMJ Open. 2013; 3.

37. Lok AS, McMahon BJ, Brown RS Jr, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology. 2016; 63:284–306. PMID:
26566246.
38. Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013; 38:98–106. PMID:
23713520.

39. Choi J, Han S, Kim N, Lim Y. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology. 2017; 66:1454–1463. PMID:
28628942.

40. Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010; 8:280–288.e1. PMID:
19948249.

41. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013; 158:329–337. PMID:
23460056.
42. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017; 153:996–1005.e1. PMID:
28642197.

43. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018; 68:25–32.

44. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017; 67:1204–1212. PMID:
28802876.

45. Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013; 31:3647–3655. PMID:
24002499.

46. Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2011; 33:1104–1112. PMID:
21488914.

47. Miao RY, Zhao HT, Yang HY, et al. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2010; 16:2931–2942. PMID:
20556841.

48. Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther. 2010; 32:851–858. PMID:
20659285.

49. Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016; 65:719–726. PMID:
27084592.

50. Martin B, Hennecke N, Lohmann V, et al. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014; 61:538–543. PMID:
24905492.

51. Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015; 22:983–991. PMID:
26482547.

52. Spaan M, van Oord G, Kreefft K, et al. Immunological analysis during interferon-free therapy for chronic hepatitis C virus infection reveals modulation of the natural killer cell compartment. J Infect Dis. 2016; 213:216–223. PMID:
26223768.

53. Serti E, Chepa-Lotrea X, Kim YJ, et al. Successful interferon-free therapy of chronic hepatitis C virus infection normalizes natural killer cell function. Gastroenterology. 2015; 149:190–200.e2. PMID:
25754160.

54. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016; 65:727–733. PMID:
27349488.

55. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol. 2016; 65:734–740. PMID:
27288051.
56. Cabibbo G, Petta S, Calvaruso V, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study? Aliment Pharmacol Ther. 2017; 46:688–695. PMID:
28791711.

57. Minami T, Tateishi R, Nakagomi R, et al. The impact of direct-acting antivirals on early tumor recurrence after radiofrequency ablation in hepatitis C-related hepatocellular carcinoma. J Hepatol. 2016; 65:1272–1273. PMID:
27524465.

58. Ikeda K, Kawamura Y, Kobayashi M, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci. 2017; 62:2932–2942. PMID:
28884320.

59. Singal A, Hoteit M, John B, et al. Direct acting antiviral therapy is associated with shorter time to HCC recurrence but not increased risk of recurrence. Hepatology. 2017; 66(1 Suppl):729A.
60. Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003; 10:204–209. PMID:
14738659.

61. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130:417–422. PMID:
15042359.

62. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009; 30:37–47. PMID:
19392863.

63. Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol. 2016; 50:120–133. PMID:
26583266.
64. Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004; 126:1005–1014. PMID:
15057740.

65. Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010; 53:291–297. PMID:
20483497.

66. Kim DY, Kim HJ, Jeong SE, et al. The Korean guideline for hepatocellular carcinoma surveillance. J Korean Med Assoc. 2015; 58:385–397.

67. Shim CW, Park JW, Kim SH, et al. Noncirrhotic hepatocellular carcinoma: etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol. 2017; 10:529–536.

68. Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017; 34:153–159. PMID:
28108047.

69. Dulku G, Dhillon R, Goodwin M, Cheng W, Kontorinis N, Mendelson R. The role of imaging in the surveillance and diagnosis of hepatocellular cancer. J Med Imaging Radiat Oncol. 2017; 61:171–179. PMID:
27981791.

70. Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996; 101:422–434. PMID:
8873514.

71. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID:
21374666.

72. Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med. 2015; 162:697–711. PMID:
25984845.
73. Singal AG, Nehra M, Adams-Huet B, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013; 108:425–432. PMID:
23337478.

74. Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006; 101:513–523. PMID:
16542288.
75. Aghoram R, Cai P, Dickinson JA. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2012; (9):CD002799. PMID:
22972059.

76. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018; 154:1706–1718.e1. PMID:
29425931.

77. European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943. PMID:
22424438.
78. Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009; 44(Suppl 19):119–121. PMID:
19148805.

79. Korean Liver Cancer Study Group. National Cancer Center Korea. 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015; 9:267–317. PMID:
25918260.
80. Korean Liver Cancer Study Group. National Cancer Center Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009; 15:391–423. PMID:
19783891.
81. Santagostino E, Colombo M, Rivi M, et al. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood. 2003; 102:78–82. PMID:
12649165.

82. Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011; 54:1987–1997. PMID:
22144108.

83. Wang JH, Chang KC, Kee KM, et al. Hepatocellular carcinoma surveillance at 4-vs. 12-month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol. 2013; 108:416–424. PMID:
23318478.
84. Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008; 6:1418–1424. PMID:
18848905.

85. Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992; 16:132–137. PMID:
1352268.

86. Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985; 89:259–266. PMID:
2408960.

87. Tanaka H, Iijima H, Nouso K, et al. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res. 2012; 42:376–384. PMID:
22221694.

88. Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography: a randomised study. Aliment Pharmacol Ther. 2013; 38:303–312. PMID:
23750991.
89. Kim SY, An J, Lim YS, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017; 3:456–463. PMID:
27657493.

90. Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. PMID:
25559230.

91. Hanna RF, Miloushev VZ, Tang A, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol (NY). 2016; 41:71–90. PMID:
26830614.

92. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010; 59:638–644. PMID:
19951909.

93. Khalili K, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011; 54:723–728. PMID:
21156219.
94. Terzi E, Iavarone M, Pompili M, et al. Contrast ultrasound LIRADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol. 2018; 68:485–492. PMID:
29133247.

95. Aube C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017; 37:1515–1525. PMID:
28346737.

96. Kim HD, Lim YS, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015; 148:1371–1382. PMID:
25733098.

97. Yoon SH, Lee JM, So YH, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol. 2009; 193:W482–W489. PMID:
19933622.

98. Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005; 42:27–34. PMID:
15954118.

99. Kierans AS, Kang SK, Ro-senkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016; 278:82–94. PMID:
26098460.

100. Choi SH, Byun JH, Lim YS, et al. Diagnostic criteria for hepatocellular carcinoma 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016; 64:1099–1107. PMID:
26820629.

101. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–2868. PMID:
25773941.

102. Ahn SJ, Choi JY, Kim KA, et al. Focal eosinophilic infiltration of the liver: gadoxetic acid-enhanced magnetic resonance imaging and diffusion-weighted imaging. J Comput Assist Tomogr. 2011; 35:81–85. PMID:
21160434.
103. Kudo M, Matsui O, Izumi N, et al. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014; 87(Suppl 1):7–21.

104. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017; 11:317–370. PMID:
28620797.

105. Yu MH, Kim JH, Yoon JH, et al. Small (</=1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014; 271:748–760. PMID:
24588677.
106. Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011; 196:W758–W765. PMID:
21606265.

107. Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012; 264:761–770. PMID:
22843769.

108. Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (<=2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013; 54:127–136. PMID:
23148300.
109. Jang KM, Kim SH, Kim YK, Choi D. Imaging features of subcentimeter hypointense nodules on gadoxetic acid-enhanced hepatobiliary phase MR imaging that progress to hypervascular hepatocellular carcinoma in patients with chronic liver disease. Acta Radiol. 2015; 56:526–535. PMID:
24838304.

110. Khalili K, Kim TK, Jang HJ, Yazdi LK, Guindi M, Sherman M. Indeterminate 1–2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011; 54:2048–2054. PMID:
22057624.

111. Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis. 2010; 30:17–25. PMID:
20175030.

112. Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008; 47:97–104. PMID:
18069697.

113. Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007; 33:437–447. PMID:
17512669.
114. Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008; 57:1592–1596. PMID:
18669577.

115. Tremosini S, Forner A, Boix L, et al. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut. 2012; 61:1481–1487. PMID:
22287594.

116. Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int. 2008; 2:17–30. PMID:
19669276.

117. Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis-hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015; 19:309–323. PMID:
25921665.
118. Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010; 138:493–502. PMID:
19852963.
119. Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000; 154:178–186. PMID:
10931690.

120. Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003; 160:381–407. PMID:
12968934.

121. Sont WN, Zielinski JM, Ashmore JP, et al. First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol. 2001; 153:309–318. PMID:
11207146.

122. Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005; 331:77. PMID:
15987704.

123. Gilbert ES. Invited commentary: studies of workers exposed to low doses of radiation. Am J Epidemiol. 2001; 153:319–322. PMID:
11207147.

124. Upton AC. National Council on Radiation Protection and Measurements Scientific Committee 1-6. The state of the art in the 1990's: NCRP Report No. 136 on the scientific bases for linearity in the dose-response relationship for ionizing radiation. Health Phys. 2003; 85:15–22. PMID:
12852466.

125. Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008; 248:995–1003. PMID:
18710988.

126. National Research Council. Committee to Assess Health Risks from Exposure to Low Levels of ionizing Radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academy Press;2006.
127. The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007; 37:1–332.
128. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011; 261:193–198. PMID:
21771956.

129. Takahashi H, Okada M, Hyodo T, et al. Can low-dose CT with iterative reconstruction reduce both the radiation dose and the amount of iodine contrast medium in a dynamic CT study of the liver? Eur J Radiol. 2014; 83:684–691. PMID:
24418284.

130. Pregler B, Beyer LP, Teufel A, et al. Low tube voltage liver MDCT with sinogram-affirmed iterative reconstructions for the detection of hepatocellular carcinoma. Sci Rep. 2017; 7:9460. PMID:
28842662.

131. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006; 44:217–231. PMID:
16298014.
132. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37:429–442. PMID:
12540794.

133. Meier V, Ramadori G. Clinical staging of hepatocellular carcinoma. Dig Dis. 2009; 27:131–141. PMID:
19546551.

134. Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002; 24:395–403. PMID:
12479938.

135. Liver Cancer Study Group of Japan. The general rules of the clinical and pathological study of primary liver cancer. 4th ed. Tokyo: Kanehara;2010.
136. Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236. PMID:
16250051.

137. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014; 146:1691–1700.e3. PMID:
24583061.

138. Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011; 117:4475–4483. PMID:
21437884.
139. National Comprehensive Cancer Network (NCCN). Hepatobiliary Cancers (version 1.2017). Fort Washington, MD: NCCN;2017.
140. Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008; 49:1912–1921. PMID:
18997056.

141. Lee JE, Jang JY, Jeong SW, et al. Diagnostic value for extrahepatic metastases of hepatocellular carcinoma in positron emission tomography/computed tomography scan. World J Gastroenterol. 2012; 18:2979–2987. PMID:
22736922.

142. Sugiyama M, Sakahara H, Torizuka T, et al. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004; 39:961–968. PMID:
15549449.
143. Cho Y, Lee DH, Lee YB, et al. Does 18F-FDG positron emission tomography-computed tomography have a role in initial staging of hepatocellular carcinoma? PLoS One. 2014; 9:e105679. PMID:
25153834.

144. Lang H, Sotiropoulos GC, Domland M, et al. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005; 92:198–202. PMID:
15609381.

145. Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004; 10:S64–S68. PMID:
14762842.

146. Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001; 234:63–70. PMID:
11420484.

147. Andreou A, Vauthey JN, Cherqui D, et al. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013; 17:66–77. PMID:
22948836.

148. Huang J, Zhang Y, Peng Z, et al. A modified TNM-7 staging system to better predict the survival in patients with hepatocellular carcinoma after hepatectomy. J Cancer Res Clin Oncol. 2013; 139:1709–1719. PMID:
23982274.

149. Lee EC, Kim SH, Park H, Lee SD, Lee SA, Park SJ. Survival analysis after liver resection for hepatocellular carcinoma: a consecutive cohort of 1002 patients. J Gastroenterol Hepatol. 2017; 32:1055–1063. PMID:
27797420.

150. Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013; 257:929–937. PMID:
23426336.
151. Kim JH, Choi DW, Kim SB. Saftey and long-term outcome following major hepatectomy for hepatocellular carcinoma combined with compensated liver cirrhosis. J Korean Surg Soc. 2006; 70:444–450.
152. Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003; 238:703–710. PMID:
14578733.

153. Finkelstein SD, Marsh W, Demetris AJ, et al. Microdissection-based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003; 37:871–879. PMID:
12668980.

154. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–207. PMID:
12547409.

155. Kaibori M, Ishizaki M, Matsui K, Kwon AH. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010; 102:462–468. PMID:
20872949.

156. Li SH, Guo ZX, Xiao CZ, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev. 2013; 14:4759–4763. PMID:
24083739.

157. Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009; 249:799–805. PMID:
19387322.

158. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006; 243:229–235. PMID:
16432356.
159. Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009; 51:890–897. PMID:
19747749.

160. Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol. 2011; 37:521–525. PMID:
21531111.

161. Kim YI, Kim HS, Park JW. Higher ratio of serum alpha-fetoprotein could predict outcomes in patients with hepatitis B virus-associated hepatocellular carcinoma and normal alanine aminotransferase. PLoS One. 2016; 11:e0157299. PMID:
27304617.

162. Kim DY, Paik YH, Ahn SH, et al. PIVKA-II is a useful tumor marker for recurrent hepatocellular carcinoma after surgical resection. Oncology. 2007; 72(Suppl 1):52–57. PMID:
18087182.

163. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973; 60:646–649. PMID:
4541913.

164. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655. PMID:
7165009.

165. Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000; 191:38–46. PMID:
10898182.
166. Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999; 229:210–215. PMID:
10024102.
167. Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004; 10:S46–S52. PMID:
14762839.

168. Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995; 130:198–203. PMID:
7848092.

169. Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999; 30:1434–1440. PMID:
10573522.

170. An M, Park JW, Shin JA, et al. The adverse effect of indirectly diagnosed portal hypertension on the complications and prognosis after hepatic resection of hepatocellular carcinoma. Korean J Hepatol. 2006; 12:553–561. PMID:
17237634.
171. Choi GH, Park JY, Hwang HK, et al. Predictive factors for longterm survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int. 2011; 31:485–493. PMID:
21382158.

172. Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006; 30:992–999. PMID:
16736327.

173. Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009; 250:922–928. PMID:
19855258.

174. He W, Zeng Q, Zheng Y, et al. The role of clinically significant portal hypertension in hepatic resection for hepatocellular carcinoma patients: a propensity score matching analysis. BMC Cancer. 2015; 15:263. PMID:
25886495.

175. Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008; 134:1908–1916. PMID:
18549877.

176. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012; 29:6–17. PMID:
22441614.

177. Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012; 256:706–712. PMID:
23095613.

178. Hu H, Han H, Han XK, Wang WP, Ding H. Nomogram for individualised prediction of liver failure risk after hepatectomy in patients with resectable hepatocellular carcinoma: the evidence from ultrasound data. Eur Radiol. 2018; 28:877–885. PMID:
28779402.

179. Kim SU, Ahn SH, Park JY, et al. Prediction of postoperative hepatic insufficiency by liver stiffness measurement (FibroScan((R))) before curative resection of hepatocellular carcinoma: a pilot study. Hepatol Int. 2008; 2:471–477. PMID:
19669322.

180. Wong JS, Wong GL, Chan AW, et al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg. 2013; 257:922–928. PMID:
23001077.

181. Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008; 18:457–467. PMID:
18058107.

182. Kim SH, Kim SH, Lee J, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009; 192:1675–1681. PMID:
19457834.

183. Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2012; 81:2417–2422. PMID:
21899970.

184. Koneru B, Teperman LW, Manzarbeitia C, et al. A multicenter evaluation of utility of chest computed tomography and bone scans in liver transplant candidates with stages I and II hepatoma. Ann Surg. 2005; 241:622–628. PMID:
15798464.

185. Liu L, Wang Z, Jiang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013; 8:e64261. PMID:
23741309.

186. Tsujita E, Taketomi A, Kitagawa D, et al. Selective hepatic vascular exclusion for the hepatic resection of HCC. Hepatogastroenterology. 2007; 54:527–530. PMID:
17523313.
187. Cucchetti A, Qiao GL, Cescon M, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014; 155:512–521. PMID:
24439747.

188. Ishii M, Mizuguchi T, Kawamoto M, et al. Propensity score analysis demonstrated the prognostic advantage of anatomical liver resection in hepatocellular carcinoma. World J Gastroenterol. 2014; 20:3335–3342. PMID:
24696614.

189. Kaibori M, Kon M, Kitawaki T, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2017; 24:616–626. PMID:
28887834.

190. Kudo A, Tanaka S, Ban D, et al. Anatomic resection reduces the recurrence of solitary hepatocellular carcinoma </=5 cm without macrovascular invasion. Am J Surg. 2014; 207:863–869. PMID:
24112679.
191. Sakoda M, Ueno S, Iino S, et al. Survival benefits of small anatomical resection of the liver for patients with hepatocellular carcinoma and impaired liver function, based on new-era imaging studies. J Cancer. 2016; 7:1029–1036. PMID:
27326244.

192. Zhao H, Chen C, Gu S, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol. 2017; 32:870–878. PMID:
27671209.
193. Huang X, Lu S. A Meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB (Oxford). 2017; 19:843–849. PMID:
28739076.

194. Feng X, Su Y, Zheng S, et al. A double blinded prospective randomized trial comparing the effect of anatomic versus non-anatomic resection on hepatocellular carcinoma recurrence. HPB (Oxford). 2017; 19:667–674. PMID:
28499749.

195. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007; 245:36–43. PMID:
17197963.
196. Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008; 143:469–475. PMID:
18374043.

197. Suh KS. Systematic hepatectomy for small hepatocellular carcinoma in Korea. J Hepatobiliary Pancreat Surg. 2005; 12:365–370. PMID:
16258804.

198. Wakai T, Shirai Y, Sakata J, et al. Anatomic resection independently improves long-term survival in patients with T1–T2 hepatocellular carcinoma. Ann Surg Oncol. 2007; 14:1356–1365. PMID:
17252289.

199. Kim IS, Lim YS, Yoon HK, et al. The effect of preoperative transarterial chemoembolization on the patient's outcome in resectable hepatocellular carcinoma. Korean J Med. 2005; 69:614–621.
200. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'eng FK. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995; 82:122–126. PMID:
7881929.

201. Hayashi H, Beppu T, Okabe H, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015; 157:20–26. PMID:
25482462.

202. Nishio T, Taura K, Koyama Y, et al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery. 2016; 159:399–408. PMID:
26209567.

203. Beppu T, Okabe H, Okuda K, et al. Portal vein embolization followed by right-side hemihepatectomy for hepatocellular carcinoma patients: a Japanese multi-institutional study. J Am Coll Surg. 2016; 222:1138–1148.e2. PMID:
27107976.

204. Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015; 157:690–698. PMID:
25704417.

205. Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg. 2015; 262:780–785. PMID:
26583666.
206. Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001; 193:109–111. PMID:
11442247.

207. Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006; 244:194–203. PMID:
16858181.
208. Jin B, Chen MT, Fei YT, Du SD, Mao YL. Safety and efficacy for laparoscopic versus open hepatectomy: a meta-analysis. Surg Oncol. 2017; 27:A26–A34. PMID:
28687154.

209. Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, et al. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study). Br J Surg. 2017; 104:525–535. PMID:
28138958.
210. Cherqui D. Laparoscopic liver resection: a new paradigm in the management of hepatocellular carcinoma? J Hepatol. 2015; 63:540–542. PMID:
26144660.

211. Takahara T, Wakabayashi G, Konno H, et al. Comparison of laparoscopic major hepatectomy with propensity score matched open cases from the National Clinical Database in Japan. J Hepatobiliary Pancreat Sci. 2016; 23:721–734. PMID:
27685870.

212. Chana P, Burns EM, Arora S, Darzi AW, Faiz OD. A systematic review of the impact of dedicated emergency surgical services on patient outcomes. Ann Surg. 2016; 263:20–27. PMID:
26840649.

213. Chen PD, Wu CY, Hu RH, et al. Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann Surg Oncol. 2017; 24:1021–1028. PMID:
27778128.

214. Lai EC, Tang CN. Long-term survival analysis of robotic versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: a comparative study. Surg Laparosc Endosc Percutan Tech. 2016; 26:162–166. PMID:
27031650.
215. Hwang S, Lee YJ, Kim KH, et al. Long-term outcome after resection of huge hepatocellular carcinoma >/= 10 cm: single-institution experience with 471 patients. World J Surg. 2015; 39:2519–2528. PMID:
26126423.
216. Zhou YM, Li B, Xu DH, Yang JM. Safety and efficacy of partial hepatectomy for huge (>/=10 cm) hepatocellular carcinoma: a systematic review. Med Sci Monit. 2011; 17:RA76–RA83. PMID:
21358616.
217. Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003; 113:495–506. PMID:
12757710.
218. Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007; 204:854–862. PMID:
17481498.

219. Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer. 2013; 4:635–643. PMID:
24155775.

220. Chan WH, Hung CF, Pan KT, et al. Impact of spontaneous tumor rupture on prognosis of patients with T4 hepatocellular carcinoma. J Surg Oncol. 2016; 113:789–795. PMID:
27062288.

221. Sada H, Ohira M, Kobayashi T, Tashiro H, Chayama K, Ohdan H. An analysis of surgical treatment for the spontaneous rupture of hepatocellular carcinoma. Dig Surg. 2016; 33:43–50. PMID:
26580332.

222. Schwarz L, Bubenheim M, Zemour J, et al. Bleeding recurrence and mortality following interventional management of spontaneous HCC rupture: results of a multicenter European study. World J Surg. 2018; 42:225–232. PMID:
28799103.

223. Aoki T, Kokudo N, Matsuyama Y, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014; 259:532–542. PMID:
23478524.
224. Li J, Huang L, Liu CF, et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol. 2014; 20:9121–9127. PMID:
25083085.
225. Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion: a Japanese nationwide survey. J Clin Oncol. 2016; 34(15_Suppl):4067.

226. Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017; 66:510–517. PMID:
28437844.

227. Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol. 2016; 22:160–167. PMID:
27044767.

228. Moon DB, Hwang S, Wang HJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg. 2013; 37:443–451. PMID:
23188531.

229. Koneru B, Cassavilla A, Bowman J, Iwatsuki S, Starzl TE. Liver transplantation for malignant tumors. Gastroenterol Clin North Am. 1988; 17:177–193. PMID:
2839423.

230. Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991; 214:221–228. PMID:
1656903.

231. Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993; 218:145–151. PMID:
8393649.

232. Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999; 19:311–322. PMID:
10518310.

233. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699. PMID:
8594428.

234. Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011; 17(Suppl 2):S44–S57. PMID:
21695773.

235. European Liver Transplant Registry (ELTR). Villejuif: ELTR;2014. Available from:
http://www.eltr.org.
237. Germani G, Gurusamy K, Garcovich M, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume. Liver Transpl. 2011; 17(Suppl 2):S58–S66. PMID:
21584928.

238. Sugimachi K, Shirabe K, Taketomi A, et al. Prognostic significance of preoperative imaging in recipients of living donor liver transplantation for hepatocellular carcinoma. Transplantation. 2011; 91:570–574. PMID:
21343876.

239. Lee JM, Trevisani F, Vilgrain V, Wald C. Imaging diagnosis and staging of hepatocellular carcinoma. Liver Transpl. 2011; 17(Suppl 2):S34–S43.

240. Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012; 13:e11–e22. PMID:
22047762.

241. Elwir S, Lake J. Current status of liver allocation in the United States. Gastroenterol Hepatol (N Y). 2016; 12:166–170. PMID:
27231445.
244. Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006; 6:1416–1421. PMID:
16686765.

245. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403. PMID:
11391528.

246. Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002; 50:123–128. PMID:
11772979.

247. Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004; 240:900–909. PMID:
15492574.
248. Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010; 16:262–278. PMID:
20209641.

249. Lee MW, Raman SS, Asvadi NH, et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysis. Hepatology. 2017; 65:1979–1990. PMID:
28170115.

250. Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002; 8:873–883. PMID:
12360427.

251. Yao FY, Kerlan RK Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008; 48:819–827. PMID:
18688876.

252. Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010; 10:129–137. PMID:
20070666.

253. Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multi-center HCC transplant consortium. Ann Surg. 2017; 266:525–535. PMID:
28654545.
254. Decaens T, Roudot-Thoraval F, Bresson-Hadni S, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005; 11:767–775. PMID:
15973710.

255. Lesurtel M, Mlhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006; 6:2644–2650. PMID:
16939518.

256. Pelletier SJ, Fu S, Thyagarajan V, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009; 15:859–868. PMID:
19642139.

257. Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. PMID:
22158026.

258. Si T, Chen Y, Ma D, et al. Transarterial chemoembolization prior to liver transplantation for patients with hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2017; 32:1286–1294. PMID:
28085213.

259. Porrett PM, Pet-erman H, Rosen M, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006; 12:665–673. PMID:
16482577.

260. Xing MZ, Kim HS. Independent prognostic factors for posttransplant survival in hepatocellular carcinoma patients undergoing liver transplantation. Cancer Medicine. 2017; 6:26–35. PMID:
27860456.

261. Mehta N, Heimbach J, Lee D, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation. 2017; 101:2071–2078. PMID:
28353492.
262. Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg. 2017; 402:863–871. PMID:
28755240.

263. Chapman WC, Garcia-Aroz S, Vachharajani N, et al. Liver transplantation for advanced hepatocellular carcinoma after downstaging without up-front stage restrictions. J Am Coll Surg. 2017; 224:610–621. PMID:
28069527.

264. Kim JH, Sinn DH, Gwak GY, et al. Factors determining long-term outcomes of hepatocellular carcinoma within the Milan criteria: liver transplantation versus locoregional therapy: a retrospective cohort study. Medicine (Baltimore). 2016; 95:e4735. PMID:
27583916.
265. Massarollo PC, Coppini AZ, Salzedas-Netto AA, Coelho FF, Minami T, Gonzalez AM. Favorable long-term outcome in patients submitted to liver transplantation after downstaging of hepatocellular carcinoma according to a Brazilian selection protocol. Transplant Proc. 2016; 48:2338–2340. PMID:
27742292.

266. Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008; 8:2547–2557. PMID:
19032223.

267. Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015; 61:1968–1977. PMID:
25689978.

268. Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008; 248:617–625. PMID:
18936575.

269. De Luna W, Sze DY, Ahmed A, et al. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009; 9:1158–1168. PMID:
19344435.

270. Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002; 235:533–539. PMID:
11923610.

271. Yao FY, Hirose R, LaBerge JM, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005; 11:1505–1514. PMID:
16315294.

272. Bhoori S, Sposito C, Germini A, Coppa J, Mazzaferro V. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int. 2010; 23:712–722. PMID:
20492616.

273. Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009; 9:1920–1928. PMID:
19552767.

274. Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl. 2015; 21:1142–1152. PMID:
25981135.

275. Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatments for hepatocellular carcinoma: results of a survey by the Italian Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2014; 37:438–444. PMID:
23719667.

276. Korean Organ Donation Agency (KODA). Bridge for life: KODA annual report. Seoul: KODA;2017.
277. Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013; 27:140–147. PMID:
23157398.

278. Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012; 18:1226–1236. PMID:
22685095.

279. Azoulay D, Audureau E, Bhangui P, et al. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg. 2017; 266:1035–1044. PMID:
27617853.
280. Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011; 53:1570–1579. PMID:
21520172.

281. Kulik LM, Fisher RA, Rodrigo DR, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant. 2012; 12:2997–3007. PMID:
22994906.

282. Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004; 127:S277–S282. PMID:
15508095.

283. Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology. 2001; 33:1073–1079. PMID:
11343234.

284. Kwon CH, Kim DJ, Han YS, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis. 2007; 25:313–319. PMID:
17960066.

285. Suh KS, Cho EH, Lee HW, et al. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis. 2007; 25:329–333. PMID:
17960068.

286. Choi HJ, Kim DG, Na GH, Han JH, Hong TH, You YK. Clinical outcome in patients with hepatocellular carcinoma after living-donor liver transplantation. World J Gastroenterol. 2013; 19:4737–4744. PMID:
23922471.

287. Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008; 14:935–945. PMID:
18581465.

288. Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007; 25:310–312. PMID:
17960065.

289. Ito T, Takada Y, Ueda M, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007; 13:1637–1644. PMID:
18044766.

290. Taketomi A, Sanefuji K, Soejima Y, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009; 87:531–537. PMID:
19307789.

291. Todo S, Furukawa H, Tada M;. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2007; 13:S48–S54. PMID:
17969069.

292. Lee JH, Cho Y, Kim HY, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann Surg. 2016; 263:842–850. PMID:
26779979.

293. Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012; 143:986–994.e3. PMID:
22750200.
294. Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017; 66:552–559. PMID:
27899297.

295. Kim SH, Kim YK. Improving outcomes of living-donor right hepatectomy. Br J Surg. 2013; 100:528–534. PMID:
23288584.

296. Kim SJ, Na GH, Choi HJ, Yoo YK, Kim DG. Surgical outcome of right liver donors in living donor liver transplantation: single-center experience with 500 cases. J Gastrointest Surg. 2012; 16:1160–1170. PMID:
22426687.

297. Shin M, Song S, Kim JM, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation. 2012; 93:942–948. PMID:
22357173.
298. Kim KH, Jung DH, Park KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg. 2011; 98:1302–1308. PMID:
21717424.

299. Hwang S, Lee SG, Lee YJ, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006; 12:920–927. PMID:
16721780.

300. Yi NJ, Suh KS, Cho JY, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl. 2007; 13:797–806. PMID:
17539000.

301. Lee JG, Lee KW, Kwon CH, et al. Donor safety in living donor liver transplantation: the Korean organ transplantation registry study. Liver Transpl. 2017; 23:999–1006. PMID:
28431203.

302. Chan SC, Chan AC, Sharr WW, et al. Perpetuating proficiency in donor right hepatectomy for living donor liver transplantation. Asian J Surg. 2013; 37:65–72. PMID:
24210956.

303. Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008; 135:468–476. PMID:
18505689.

304. Siegler M, Simmerling MC, Siegler JH, Cronin DC 2nd. Recipient deaths during donor surgery: a new ethical problem in living donor liver transplantation (LDLT). Liver Transpl. 2006; 12:358–360. PMID:
16498653.

305. Brown RS Jr. Live donors in liver transplantation. Gastroenterology. 2008; 134:1802–1813. PMID:
18471556.

306. Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol. 2005; 43:584–589. PMID:
16120468.

307. Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012; 18:62–69. PMID:
21964956.

308. Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005; 234:961–967. PMID:
15665226.

309. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004; 127:1714–1723. PMID:
15578509.
310. Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005; 129:122–130. PMID:
16012942.

311. Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003; 228:235–240. PMID:
12759473.

312. Kim YS, Lim HK, Rhim H, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013; 58:89–97. PMID:
23023009.

313. Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89. PMID:
18008357.

314. Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017; 3:CD011650. PMID:
28351116.
315. Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017; 104:1775–1784. PMID:
29091283.

316. Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006; 243:321–328. PMID:
16495695.

317. Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012; 57:794–802. PMID:
22634125.

318. Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010; 252:903–912. PMID:
21107100.

319. Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Radiology. 2018; 287:461–472. PMID:
29135366.

320. Qi X, Tang Y, An D, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2014; 48:450–457. PMID:
24172183.
321. Jia JB, Zhang D, Ludwig JM, Kim HS. Radiofrequency ablation versus resection for hepatocellular carcinoma in patients with Child-Pugh A liver cirrhosis: a meta-analysis. Clin Radiol. 2017; 72:1066–1075. PMID:
28851491.

322. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010; 51:1284–1290. PMID:
20099299.

323. Lee HW, Lee JM, Yoon JH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018; 94:74–82. PMID:
29441336.

324. Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014; 271:909–918. PMID:
24520944.

325. Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection— propensity score analyses of long-term outcomes. Radiology. 2015; 275:908–919. PMID:
25688888.
326. Kim GA, Shim J, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg. 2016; 103:126–135. PMID:
26572697.

327. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009; 252:905–913. PMID:
19567647.

328. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010; 116:5452–5460. PMID:
20672352.
329. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013; 31:426–432. PMID:
23269991.

330. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013; 25:187–194. PMID:
23134976.
331. Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016; 17:93–102. PMID:
26798221.

332. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013; 19:3872–3882. PMID:
23840128.

333. de Baere T, Risse O, Kuoch V, et al. Adverse events during radio-frequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003; 181:695–700. PMID:
12933462.
334. Rhim H, Yoon KH, Lee JM, et al. Major complications after radiofrequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003; 23:123–134. PMID:
12533647.

335. Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radio-frequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009; 19:2630–2640. PMID:
19557416.

336. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for percutaneous radiofrequency ablation of small hepatocellular carcinomas (1–3 cm): value of fusion imaging with conventional US and CT/MR images. J Vasc Interv Radiol. 2013; 24:958–965. PMID:
23796082.

337. Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010; 78(Suppl 1):40–45.

338. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005; 54:1151–1156. PMID:
16009687.

339. Ishii H, Okada S, Nose H, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996; 77:1792–1796. PMID:
8646676.

340. Vilana R, Bruix J, Bru C, Ayuso C, Solé M, Rodés J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992; 16:353–357. PMID:
1322349.

341. Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: a study on 207 patients. Cancer. 1992; 69:925–929. PMID:
1310435.
342. Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol. 2008; 43:727–735. PMID:
18569991.

343. Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer research. 2011; 31:2291–2295. PMID:
21737654.
344. Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009; 49:453–459. PMID:
19065676.

345. Shen A, Zhang H, Tang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013; 28:793–800. PMID:
23432154.
346. Yang B, Zan RY, Wang SY, et al. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2015; 13:96. PMID:
25889181.

347. Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017; 15:126. PMID:
28693505.

348. Ebara M, Okabe S, Kita K, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005; 43:458–464. PMID:
16005538.

349. Cha DI, Lee MW, Rhim H, Choi D, Kim YS, Lim HK. Therapeutic efficacy and safety of percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas in high risk locations. Korean J Radiol. 2013; 14:240–247. PMID:
23483664.

350. Lencioni R, Llovet JM. Percutaneous ethanol injection for hepatocellular carcinoma: alive or dead? J Hepatol. 2005; 43:377–380. PMID:
16005537.

351. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011; 258:351–369. PMID:
21273519.

352. Shi Y, Zhai B. A recent advance in image-guided locoregional therapy for hepatocellular carcinoma. Gastrointest Tumors. 2016; 3:90–102. PMID:
27904861.

353. Yu J, Yu XL, Han ZY, et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017; 66:1172–1173. PMID:
27884919.

354. Vietti VN, Duran R, Guiu B, et al. Microwave ablation and radiofrequency ablation for the treatment of hepatocellular carcinoma: result of the first prospective randomized controlled trial. Ann Oncol. 2017; 28(Suppl_3):PD-014.

355. Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015; 61:1579–1590. PMID:
25284802.

356. Sotiropoulos GC, Lang H, Frilling A, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology. 2006; 53:322–329. PMID:
16795964.
357. Kwak HW, Park JW, Nam BH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2014; 29:820–829. PMID:
24325272.

358. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015; 35:2155–2166. PMID:
25752327.
359. Gaba RC. Chemoembolization practice patterns and technical methods among interventional radiologists: results of an online survey. AJR Am J Roentgenol. 2012; 198:692–699. PMID:
22358011.

360. Satake M, Uchida H, Arai Y, et al. Transcatheter arterial chemoembolization (TACE) with lipiodol to treat hepatocellular carcinoma: survey results from the TACE study group of Japan. Cardiovasc Intervent Radiol. 2008; 31:756–761. PMID:
18389187.

361. Brown DB, Gould JE, Gervais DA, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:S425–S434. PMID:
19560030.

362. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004; 127:S179–S188. PMID:
15508083.

363. Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993; 188:79–83. PMID:
8390073.

364. Golfieri R, Cappelli A, Cucchetti A, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (< 5 cm) hepatocellular carcinomas. Hepatology. 2011; 53:1580–1589. PMID:
21351114.
365. Golfieri R, Ren-zulli M, Mosconi C, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013; 24:509–517. PMID:
23428355.

366. Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012; 81:3985–3992. PMID:
22959287.

367. Miyayama S, Yamashiro M, Hashimoto M, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma </=6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014; 37:388–395. PMID:
23775550.
368. Pung L, Ahmad M, Mueller K, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol. 2017; 28:334–341. PMID:
28109724.

369. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739. PMID:
12049862.

370. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002; 35:1164–1171. PMID:
11981766.

371. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016; 64:106–116. PMID:
26765068.

372. Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006; 131:461–469. PMID:
16890600.

373. Ikeda M, Arai Y, Park SJ, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013; 24:490–500. PMID:
23466316.

374. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380. PMID:
28130846.

375. Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011; 258:627–634. PMID:
21273524.

376. Georgiades CS, Hong K, D'Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005; 16:1653–1659. PMID:
16371532.

377. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction: a prospective controlled study. Cancer. 1997; 79:2087–2094. PMID:
9179054.
378. Silva JP, Berger NG, Tsai S, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford). 2017; 19:659–666. PMID:
28552299.

379. Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995; 165:315–321. PMID:
7618547.

380. Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011; 18:413–420. PMID:
20839057.

381. Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012; 29:2992–2997. PMID:
22200992.

382. Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013; 13:60. PMID:
23566041.

383. Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015; 26:320–329.e6. PMID:
25612807.

384. Jung SM, Jang JW, You CR, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol. 2012; 27:684–689. PMID:
21916984.

385. Lee IC, Huo TI, Huang YH, et al. Transarterial chemoembolization can prolong survival for patients with metastatic hepatocellular carcinoma: a propensity score matching analysis. Hepatol Int. 2012; 6:753–762. PMID:
26201524.

386. Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011; 26:145–154. PMID:
21175808.

387. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669. PMID:
29543938.
388. Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007; 18:365–376. PMID:
17377182.

389. Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol. 2002; 20:4459–4465. PMID:
12431969.

390. Bargellini I, Sacco R, Bozzi E, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012; 81:1173–1178. PMID:
21466931.

391. Chung JW, Park JH, Han JK, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996; 198:33–40. PMID:
8539401.

392. Ogasawara S, Chiba T, Ooka Y, et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018; 67:575–585. PMID:
28746788.

393. Yang H, Seon J, Sung PS, et al. Dexamethasone prophylaxis to alleviate postembolization syndrome after transarterial chemoembolization for hepatocellular carcinoma: a randomized, double-blinded, placebo-controlled study. J Vasc Interv Radiol. 2017; 28:1503–1511.e2. PMID:
28941589.

394. Lv N, Kong Y, Mu L, Pan T, Xie Q, Zhao M. Effect of perioperative parecoxib sodium on postoperative pain control for transcatheter arterial chemoembolization for inoperable hepatocellular carcinoma: a prospective randomized trial. Eur Radiol. 2016; 26:3492–3499. PMID:
26801163.

395. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007; 46:474–481. PMID:
17239480.

396. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–52. PMID:
19908093.

397. Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011; 22:1545–1552. PMID:
21849247.

398. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014; 111:255–264. PMID:
24937669.

399. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016; 48:571–577. PMID:
26965785.

400. Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads: implications for clinical practice and trial design. J Hepatol. 2012; 56:1330–1335. PMID:
22314428.

401. Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012; 35:1119–1128. PMID:
22614031.

402. Lee M, Chung JW, Lee KH, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017; 28:502–512. PMID:
27856136.

403. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006; 17:1251–1278. PMID:
16923973.
404. Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013; 57:1826–1837. PMID:
22911442.

405. Kim DY, Park BJ, Kim YH, et al. Radioembolization with yttrium-90 resin microspheres in hepatocellular carcinoma: a multicenter prospective study. Am J Clin Oncol. 2015; 38:495–501. PMID:
24064753.
406. Chow PK, Gandhi M. Phase III multicenter open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: the SIRveNIB study. J Clin Oncol. 2017; 35(15_Suppl):4002.
407. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017; 18:1624–1636. PMID:
29107679.
408. Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016; 151:1155–1163.e2. PMID:
27575820.
409. Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015; 35:1715–1721. PMID:
25443863.

410. Zhang Y, Li Y, Ji H, Zhao X, Lu H. Transarterial Y90 radioembolization versus chemoembolization for patients with hepatocellular carcinoma: a meta-analysis. Biosci Trends. 2015; 9:289–298. PMID:
26559021.

411. Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs. chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016; 8:770–777. PMID:
27366304.
412. Lobo L, Yakoub D, Picado O, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2016; 39:1580–1588. PMID:
27586657.

413. Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2015; 38:352–360. PMID:
25373796.

414. Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010; 138:52–64. PMID:
19766639.

415. Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011; 54:868–878. PMID:
21618574.

416. Cha H, Park HC, Yu JI, et al. Clinical practice patterns of radiotherapy in patients with hepatocellular carcinoma: a Korean Radiation Oncology Group Study (KROG 14-07). Cancer Res Treat. 2017; 49:61–69. PMID:
27338036.

417. Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure. Cancer. 2006; 106:1653–1663. PMID:
16541431.
418. Kim TH, Kim DY, Park JW, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007; 67:225–231. PMID:
17056199.

419. Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005; 62:1371–1378. PMID:
16029795.

420. Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010; 76:S94–S100. PMID:
20171524.

421. Culleton S, Jiang H, Haddad CR, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014; 111:412–417. PMID:
24906626.

422. Feng M, Suresh K, Schipper MJ, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018; 4:40–47. PMID:
28796864.
423. Mizumoto M, Okumura T, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011; 81:1039–1045. PMID:
20888707.

424. Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011; 81:e447–e453. PMID:
21645977.

425. Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012; 84:355–361. PMID:
22342300.

426. Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012; 118:5424–5431. PMID:
22570179.
427. Honda Y, Kimura T, Aikata H, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013; 28:530–536. PMID:
23216217.

428. Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014; 53:399–404. PMID:
23962244.

429. Bae SH, Kim MS, Cho CK, et al. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Korean Med Sci. 2013; 28:213–219. PMID:
23400333.

430. Yoon SM, Lim YS, Park MJ, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013; 8:e79854. PMID:
24255719.

431. Lo CH, Huang WY, Lee MS, et al. Stereotactic ablative radiotherapy for unresectable hepatocellular carcinoma patients who failed or were unsuitable for transarterial chemoembolization. Eur J Gastroenterol Hepatol. 2014; 26:345–352. PMID:
24384685.

432. Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013; 8:e63864. PMID:
23737955.

433. Weiner AA, Olsen J, Ma D, et al. Stereotactic body radiotherapy for primary hepatic malignancies: report of a phase I/II institutional study. Radiother Oncol. 2016; 121:79–85. PMID:
27566894.
434. Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016; 122:2041–2049. PMID:
27062278.

435. Wahl DR, Stenmark MH, Tao Y, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016; 34:452–459. PMID:
26628466.

436. Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005; 23:1839–1846. PMID:
15774777.

437. Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009; 74:831–836. PMID:
19304408.

438. Sugahara S, Nakayama H, Fukuda K, et al. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009; 185:782–788. PMID:
20013087.

439. Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009; 115:5499–5506. PMID:
19645024.
440. Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011; 117:4890–4904. PMID:
21495022.

441. Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011; 117:3053–3059. PMID:
21264826.

442. Kim TH, Park JW, Kim YJ, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat. 2015; 47:34–45. PMID:
25381830.

443. Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016; 34:460–468. PMID:
26668346.

444. Bush DA, Smith JC, Slater JD, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. Int J Radiat Oncol Biol Phys. 2016; 95:477–482. PMID:
27084661.

445. Kasuya G, Kato H, Yasuda S, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: combined analyses of 2 prospective trials. Cancer. 2017; 123:3955–3965. PMID:
28662297.

446. Fukuda K, Okumura T, Abei M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017; 108:497–503. PMID:
28012214.

447. Oshiro Y, Mizumoto M, Okumura T, et al. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol. 2017; 123:240–245. PMID:
28366501.

448. Kim TH, Park JW, Kim BH, et al. Optimal time of tumour response evaluation and effectiveness of hypofractionated proton beam therapy for inoperable or recurrent hepatocellular carcinoma. Oncotarget. 2018; 9:4034–4043. PMID:
29423102.

449. Su TS, Liang P, Liang J, et al. Long-term survival analysis of stereotactic ablative radiotherapy versus liver resection for small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017; 98:639–646. PMID:
28581406.

450. Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015; 1:756–765. PMID:
26182200.
451. Hsu HC, Chen TY, Chiu KW, et al. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol. 2007; 80:38–42. PMID:
16971419.

452. Oh D, Lim DH, Park HC, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010; 33:370–375. PMID:
20142728.
453. Choi C, Koom WS, Kim TH, et al. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014; 90:1051–1060. PMID:
25303890.
454. Yu JI, Park HC, Lim DH, et al. Scheduled interval trans-catheter arterial chemoembolization followed by radiation therapy in patients with unresectable hepatocellular carcinoma. J Korean Med Sci. 2012; 27:736–743. PMID:
22787367.

455. Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003; 57:113–119. PMID:
12909223.

456. Kim DY, Park W, Lim DH, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005; 103:2419–2426. PMID:
15822130.

457. Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003. PMID:
18615601.

458. Katamura Y, Aikata H, Takaki S, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol. 2009; 44:492–502. PMID:
19330281.

459. Shirai S, Sato M, Suwa K, et al. Feasibility and efficacy of single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma 8 cm or more with portal vein tumor thrombus in combination with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2010; 76:1037–1044. PMID:
19540053.

460. Koo JE, Kim JH, Lim YS, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2010; 78:180–187. PMID:
19926229.

461. Yu JI, Park HC, Lim DH, et al. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011; 26:1014–1022. PMID:
21860551.

462. Chuma M, Taguchi H, Yamamoto Y, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011; 26:1123–1132. PMID:
21501224.

463. Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012; 82:2004–2011. PMID:
21621346.

464. Hou JZ, Zeng ZC, Zhang JY, Fan J, Zhou J, Zeng MS. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys. 2012; 84:362–368. PMID:
22381903.

465. Tang QH, Li AJ, Yang GM, et al. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013; 37:1362–1370. PMID:
23456227.

466. Park MS, Kim SU, Park JY, et al. Combination treatment of localized concurrent chemoradiation therapy and transarterial chemoembolization in locally advanced hepatocellular carcinoma with intrahepatic metastasis. Cancer Chemother Pharmacol. 2013; 71:165–173. PMID:
23079897.

467. Tanaka Y, Nakazawa T, Komori S, et al. Radiotherapy for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels: efficacy and outcomes. J Gastroenterol Hepatol. 2014; 29:352–357. PMID:
23869689.

468. Yu JI, Yoon SM, Park HC, et al. Multicenter validation study of a prognostic index for portal vein tumor thrombosis in hepatocellular carcinoma. Cancer Res Treat. 2014; 46:348–357. PMID:
25036573.

469. Lee SU, Park JW, Kim TH, et al. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014; 190:806–814. PMID:
24589917.

470. Duan F, Yu W, Wang Y, et al. Trans-arterial chemoembolization and external beam radiation therapy for treatment of hepatocellular carcinoma with a tumor thrombus in the inferior vena cava and right atrium. Cancer Imaging. 2015; 15:7. PMID:
26007646.

471. Yu JI, Park JW, Park HC, et al. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: an external validation study. Radiother Oncol. 2016; 118:408–415. PMID:
26830695.

472. Kim DY, Park JW, Kim TH, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. 2017; 122:122–129. PMID:
28034460.

473. Im JH, Yoon SM, Park HC, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int. 2017; 37:90–100.

474. Zhao Q, Zhu K, Yue J, et al. Comparison of intra-arterial chemoembolization with and without radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a meta-analysis. Ther Clin Risk Manag. 2017; 13:21–31. PMID:
28053537.

475. Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014; 14:84. PMID:
24886354.

476. Cho JY, Paik YH, Park HC, et al. The feasibility of combined transcatheter arterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma. Liver Int. 2014; 34:795–801. PMID:
24350564.

477. Skinner HD, Sharp HJ, Kaseb AO, et al. Radiation treatment outcomes for unresectable hepatocellular carcinoma. Acta Oncol. 2011; 50:1191–1198. PMID:
21793641.

478. Lee HS, Choi GH, Choi JS, et al. Surgical resection after down-staging of locally advanced hepatocellular carcinoma by localized concurrent chemoradiotherapy. Ann Surg Oncol. 2014; 21:3646–3653. PMID:
24916746.

479. Li N, Feng S, Xue J, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford). 2016; 18:549–556. PMID:
27317960.

480. Hamaoka M, Kobayashi T, Kuroda S, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg. 2017; 44:223–228. PMID:
28676383.

481. Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012; 83:895–900. PMID:
22172906.

482. Mannina EM, Cardenes HR, Lasley FD, et al. Role of stereotactic body radiation therapy before orthotopic liver transplantation: retrospective evaluation of pathologic response and outcomes. Int J Radiat Oncol Biol Phys. 2017; 97:931–938. PMID:
28333015.

483. Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma: an intention-to-treat analysis. J Hepatol. 2017; 67:92–99. PMID:
28257902.

484. Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005; 61:1143–1150. PMID:
15752895.

485. Kim TH, Kim DY, Park JW, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006; 29:568–575. PMID:
17148993.

486. Seong J, Park HC, Han KH, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000; 47:1331–1335. PMID:
10889387.

487. Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012; 82:e603–e607. PMID:
22208963.

488. Dawson LA, McGinn CJ, Lawrence TS. Conformal chemoradiation for primary and metastatic liver malignancies. Semin Surg Oncol. 2003; 21:249–255. PMID:
14648782.

489. Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol. 2013; 31:3980–3986. PMID:
24062394.

490. Cheng SH, Lin YM, Chuang VP, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999; 14:1025–1033. PMID:
10530500.

491. Huang JF, Wang LY, Lin ZY, et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol. 2002; 17:190–195. PMID:
11966950.

492. Yoon SM, Kim JH, Choi EK, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004; 36:79–84. PMID:
20396570.

493. Zeng ZC, Tang ZY, Fan J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys. 2005; 63:1067–1076. PMID:
15913915.

494. Park YJ, Lim DH, Paik SW, et al. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006; 41:1099–1106. PMID:
17160521.

495. Yamashita H, Nakagawa K, Shiraishi K, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol. 2007; 22:523–527. PMID:
17376045.

496. Jang JW, Kay CS, You CR, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys. 2009; 74:412–418. PMID:
18963538.

497. Yeung R, Hamm J, Liu M, Schellenberg D. Institutional analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lymph node metastases. Radiat Oncol. 2017; 12:105. PMID:
28637480.

498. Kim Y, Park HC, Yoon SM, et al. Prognostic group stratification and nomogram for predicting overall survival in patients who received radiotherapy for abdominal lymph node metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 15-02). Oncotarget. 2017; 8:94450–94461. PMID:
29212241.

499. Jung J, Yoon SM, Park HC, et al. Radiotherapy for adrenal metastasis from hepatocellular carcinoma: a multi-institutional retrospective study (KROG 13-05). PLoS One. 2016; 11:e0152642. PMID:
27022932.

500. Jiang W, Zeng ZC, Zhang JY, et al. Palliative radiation therapy for pulmonary metastases from hepatocellular carcinoma. Clin Exp Metastasis. 2012; 29:197–205. PMID:
22173728.

501. Taki Y, Yamaoka Y, Takayasu T, et al. Bone metastases of hepatocellular carcinoma after liver resection. J Surg Oncol. 1992; 50:12–18. PMID:
1315407.

502. Murakami R, Baba Y, Furusawa M, et al. Short communication: the value of embolization therapy in painful osseous metastases from hepatocellular carcinomas; comparative study with radiation therapy. Br J Radiol. 1996; 69:1042–1044. PMID:
8958023.
503. Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998; 93:2167–2171. PMID:
9820391.

504. Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005; 25:261–265. PMID:
15780048.

505. He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009; 115:2710–2720. PMID:
19382203.

506. Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Fukushima S, Saito T. Radiation therapy and palliative care prolongs the survival of hepatocellular carcinoma patients with bone metastases. Intern Med. 2016; 55:1077–1083. PMID:
27150858.

507. Jung IH, Yoon SM, Kwak J, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. 2017; 8:15182–15192. PMID:
28146433.

508. Nakamura N, Igaki H, Yamashita H, et al. A retrospective study of radiotherapy for spinal bone metastases from hepatocellular carcinoma (HCC). Jpn J Clin Oncol. 2007; 37:38–43. PMID:
17142252.

509. Chang UK, Kim MS, Han CJ, Lee DH. Clinical result of stereotactic radiosurgery for spinal metastasis from hepatocellular carcinoma: comparison with conventional radiation therapy. J Neurooncol. 2014; 119:141–148. PMID:
24803002.

510. Rades D, Dahlke M, Janssen S, Gebauer N, Bartscht T. Radiation therapy for metastatic spinal cord compression in patients with hepatocellular carcinoma. In Vivo. 2015; 29:749–752. PMID:
26546531.
511. Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009; 91:307–313. PMID:
18949445.
512. Park Y, Kim KS, Kim K, et al. Nomogram prediction of survival in patients with brain metastases from hepatocellular carcinoma treated with whole-brain radiotherapy: a multicenter retrospective study. J Neurooncol. 2015; 125:377–383. PMID:
26342711.

513. Wang S, Wang A, Lin J, et al. Brain metastases from hepatocellular carcinoma: recent advances and future avenues. Oncotarget. 2017; 8:25814–25829. PMID:
28445959.

514. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390. PMID:
18650514.

515. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34. PMID:
19095497.

516. Cainap C, Qin S, Huang WT, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015; 33:172–179. PMID:
25488963.

517. Cheng AL, Thongprasert S, Lim HY, et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2016; 64:774–784. PMID:
27082062.

518. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013; 31:3517–3524. PMID:
23980084.
519. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–1173. PMID:
29433850.

520. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015; 33:559–566. PMID:
25547503.

521. Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008; 99:159–165. PMID:
17953709.

522. Kim JE, Ryoo BY, Ryu MH, et al. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011; 68:1285–1290. PMID:
21445543.

523. Shim JH, Park JW, Choi JI, Park BJ, Kim CM. Practical efficacy of sorafenib monotherapy for advanced hepatocellular carcinoma patients in a Hepatitis B virus-endemic area. J Cancer Res Clin Oncol. 2009; 135:617–625. PMID:
18846384.

524. Kim HY, Park JW, Joo J, et al. Worse outcome of sorafenib therapy associated with ascites and Child-Pugh score in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2013; 28:1756–1761. PMID:
23800278.

525. Kudo M, Arizumi T. Transarterial chemoembolization in combination with a molecular targeted agent: lessons learned from negative trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017; 93(Suppl 1):127–134.

526. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019; 70:684–691. PMID:
30529387.

527. Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician's perspective. Semin Oncol. 2014; 41(Suppl 2):S1–S16.

528. Granito A, Marinelli S, Negrini G, Menetti S, Benevento F, Bolondi L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap Adv Gastroenterol. 2016; 9:240–249.

529. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502. PMID:
28434648.

530. Sangro B, Melero I, Yau T, et al. Nivolumab in sorafenib-naive and -experienced patients with advanced hepatocellular carcinoma: CheckMate 040 study. In : Proceedings of the 11th Annual Conference of International Liver Cancer Association; 2017 September 15–17; Seoul, Korea.
531. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009; 137:850–855. PMID:
19524573.

532. Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991; 214:114–117. PMID:
1714267.

533. Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol. 2017; 52:624–634. PMID:
28276833.

534. Hong Y, Wu LP, Ye F, Zhou YM. Adjuvant intrahepatic injection iodine-131-lipiodol improves prognosis of patients with hepatocellular carcinoma after resection: a meta-analysis. Indian J Surg. 2015; 77:1227–1232. PMID:
27011542.

535. Riaz IB, Riaz H, Riaz T, et al. Role of vitamin K2 in preventing the recurrence of hepatocellular carcinoma after curative treatment: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2012; 12:170. PMID:
23191943.

536. Chu KJ, Lai EC, Yao XP, et al. Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation: a systematic review and meta-analysis. Asian J Surg. 2010; 33:120–126. PMID:
21163409.
537. Zhong J, Xiang B, Ma L, Li L. Conventional oral systemic chemotherapy for postoperative hepatocellular carcinoma: a systematic review. Mol Clin Oncol. 2014; 2:1091–1096. PMID:
25279203.

538. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015; 16:1344–1354. PMID:
26361969.

539. Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000; 356:802–807. PMID:
11022927.

540. Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2016; 5:e1083671. PMID:
27141337.

541. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015; 148:1383–1391.e6. PMID:
25747273.

542. Yu X, Zhao H, Liu L, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol. 2014; 34:194–203. PMID:
24337625.
543. Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009; 41:36–41. PMID:
18818130.

544. Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008; 31:63–71. PMID:
18157013.

545. Lee JH, Lee J, Lim YS, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. J Hepatol. 2018; 68(Suppl 1):S37–S38.

546. Wang H, Liu A, Bo W, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis. 2016; 48:1275–1282. PMID:
27481586.

547. Jianyong L, Jinjing Z, Lunan Y, et al. Preoperative adjuvant transarterial chemoembolization cannot improve the long term outcome of radical therapies for hepatocellular carcinoma. Sci Rep. 2017; 7:41624. PMID:
28155861.

548. Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994; 106:1618–1624. PMID:
8194710.

549. Shirabe K, Kanematsu T, Matsumata T, et al. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991; 14:802–805. PMID:
1657754.

550. Nakashima O, Kojiro M. Recurrence of hepatocellular carcinoma: multicentric occurrence or intrahepatic metastasis? A viewpoint in terms of pathology. J Hepatobiliary Pancreat Surg. 2001; 8:404–440. PMID:
11702248.

551. Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008; 100:698–711. PMID:
18477802.

552. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: longterm results of treatment and prognostic factors. Ann Surg. 1999; 229:216–222. PMID:
10024103.
553. Adachi E, Maeda T, Matsumata T, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995; 108:768–775. PMID:
7875479.

554. Kim BK, Park JY, Kim DY, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int. 2008; 28:393–401. PMID:
18028321.

555. Ohkubo K, Kato Y, Ichikawa T, et al. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer. 2002; 94:2663–2668. PMID:
12173334.

556. Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol. 2008; 103:1663–1673. PMID:
18616655.

557. Kubo S, Yamamoto T, Ikebe T, et al. Relationship between multicentric occurrence of hepatocellular carcinoma and histology of noncancerous hepatic tissue in patients with chronic hepatitis C. Jpn J Cancer Res. 1999; 90:1076–1080. PMID:
10595735.

558. Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma: a systematic review. Surg Oncol. 2013; 22:e23–e30. PMID:
23535302.
559. Chan DL, Alzahrani NA, Morris DL, Chua TC. Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2014; 29:31–41. PMID:
24117517.

560. Poon RT, Fan ST, O'Suilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg. 2002; 195:311–318. PMID:
12229937.
561. Choi D, Lim HK, Rhim H, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007; 14:2319–2329. PMID:
17522947.

562. Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology. 2012; 262:689–700. PMID:
22157201.

563. Shim JH, Kim KM, Lee YJ, et al. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2010; 17:869–877. PMID:
20033326.

564. Shimada K, Sakamoto Y, Esaki M, et al. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007; 14:2337–2347. PMID:
17503155.

565. Gavriilidis P, Askari A, Azoulay D. Survival following redo hepatectomy vs radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2017; 19:3–9. PMID:
28341429.

566. Wang DY, Liu L, Qi XS, et al. Hepatic Re-resection versus transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma after initial resection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015; 16:5573–5578. PMID:
26225712.

567. Zhang CS, Zhang JL, Li XH, Li L, Li X, Zhou XY. Is radiofrequency ablation equal to surgical re-resection for recurrent hepatocellular carcinoma meeting the Milan criteria? A meta-analysis. J BUON. 2015; 20:223–230. PMID:
25778320.
568. Cai H, Kong W, Zhou T, Qiu Y. Radiofrequency ablation versus reresection in treating recurrent hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2014; 93:e122. PMID:
25396332.
569. Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017; 104:1433–1442. PMID:
28628947.

570. Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010; 10:78. PMID:
20618937.

571. Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000; 32:269–278. PMID:
10707867.

572. Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011; 53:136–147. PMID:
20967759.

573. Imai K, Beppu T, Chikamoto A, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014; 44:E335–E345. PMID:
24552247.

574. Xie X, Jiang C, Peng Z, et al. Local recurrence after radiofrequency ablation of hepatocellular carcinoma: treatment choice and outcome. J Gastrointest Surg. 2015; 19:1466–1475. PMID:
26014717.

575. Okuwaki Y, Nakazawa T, Kokubu S, et al. Repeat radiofrequency ablation provides survival benefit in patients with intrahepatic distant recurrence of hepatocellular carcinoma. Am J Gastroenterol. 2009; 104:2747–2753. PMID:
19603009.

576. Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004; 10:534–540. PMID:
15048797.

577. Hollebecque A, Decaens T, Boleslawski E, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol. 2009; 33:361–369. PMID:
19398289.

578. Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol. 2007; 189:352–358. PMID:
17646461.

579. Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017; 266:118–125. PMID:
27433914.
580. Roh YN, David Kwon CH, Song S, et al. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2014; 28:141–148. PMID:
24372624.

581. Sapisochin G, Goldaracena N, Astete S, et al. Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series. Ann Surg Oncol. 2015; 22:2286–2294. PMID:
25472651.

582. Taketomi A, Fukuhara T, Morita K, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010; 17:2283–2289. PMID:
20204531.

583. Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016; 200:122–130. PMID:
26277218.

584. Zhou B, Shan H, Zhu KS, et al. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010; 21:333–338. PMID:
20116286.

585. Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014; 19:309–316. PMID:
24975583.

586. Sposito C, Mariani L, Germini A, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013; 59:59–66. PMID:
23500153.

587. Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int. 2012; 25:1158–1164. PMID:
22882364.

588. Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010; 52:771–775. PMID:
20347502.

589. Waghray A, Balci B, El-Gazzaz G, et al. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013; 27:555–561. PMID:
23758296.

590. Korean Liver Cancer Study G. National Cancer Center K. 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522. PMID:
25995680.
591. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011; 29:339–364. PMID:
21829027.

592. Vogl TJ, Trapp M, Schroeder H, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000; 214:349–357. PMID:
10671580.

593. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010; 52:762–773. PMID:
20564355.

594. Yamanaka K, Hatano E, Kitamura K, et al. Early evaluation of transcatheter arterial chemoembolization-refractory hepatocellular carcinoma. J Gastroenterol. 2012; 47:343–346. PMID:
22183859.

595. Park JW, Amarapurkar D, Chao Y, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC). Liver Int. 2013; 33:327–337. PMID:
23331661.

596. Kim HY, Park JW, Joo J, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012; 27:1051–1056. PMID:
22098152.

597. Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013; 57:2261–2273. PMID:
23316013.

598. Adhoute X, Penaranda G, Naude S, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015; 62:855–862. PMID:
25463541.

599. Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012; 57:821–829. PMID:
22727733.

600. Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014; 87:330–341. PMID:
25227534.

601. Takaki H, Yamakado K, Tsurusaki M, et al. Hepatic arterial infusion chemotherapy with fine-powder cisplatin and iodized-oil suspension in patients with intermediate-stage and advanced-stage (Barcelona Clinic Liver Cancer stage-B or stage-C) hepatocellular carcinoma: multicenter phase-II clinical study. Int J Clin Oncol. 2015; 20:745–754. PMID:
25432660.

602. Lin J, Wu L, Bai X, et al. Combination treatment including targeted therapy for advanced hepatocellular carcinoma. Oncotarget. 2016; 7:71036–71051. PMID:
27626176.

603. Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014; 3:9–17. PMID:
24804173.

604. Liu L, Chen H, Wang M, et al. Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One. 2014; 9:e91124. PMID:
24651044.

605. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016; 64:1090–1098. PMID:
26809111.

606. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017; 2:565–575. PMID:
28648803.
607. Kudo M, Cheng AL, Park JW, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018; 3:37–46. PMID:
28988687.

608. Reig M, Rimola J, Torres F, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013; 58:2023–2031. PMID:
23787822.

609. Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014; 68:609–617. PMID:
24283303.
610. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013; 31:3509–3516. PMID:
23980090.

611. Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014; 312:57–67. PMID:
25058218.
612. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015; 16:859–870. PMID:
26095784.

613. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018; 19:682–693. PMID:
29625879.

614. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011; 129:245–255. PMID:
21170960.

615. Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013; 12:1322–1331. PMID:
23619301.

616. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109. PMID:
15466206.

617. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66. PMID:
27932229.

618. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018; 379:54–63. PMID:
29972759.

619. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20:282–296. PMID:
30665869.

620. Brandi G, de Rosa F, Agostini V, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013; 18:1256–1257. PMID:
24232581.

621. Mir O, Coriat R, Boudou-Rouquette P, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Med Oncol. 2012; 29:2793–2799. PMID:
22427209.

622. Lee JE, Bae SH, Choi JY, Yoon SK, You YK, Lee MA. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J Gastroenterol. 2014; 20:235–241. PMID:
24415877.

623. Chlebowski RT, Brzechwa-Adjukiewicz A, Cowden A, Block JB, Tong M, Chan KK. Doxorubicin (75 mg/m2) for hepatocellular carcinoma: clinical and pharmacokinetic results. Cancer Treat Rep. 1984; 68:487–491. PMID:
6322986.
624. Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma: adriamycin versus quadruple chemotherapy. Cancer. 1984; 53:401–405. PMID:
6198058.

625. Sciarrino E, Simonetti RG, Le Moli S, Pagliaro L. Adriamycin treatment for hepatocellular carcinoma: experience with 109 patients. Cancer. 1985; 56:2751–2755. PMID:
2413981.

626. Tetef M, Doroshow J, Akman S, et al. 5-Fluorouracil and high-dose calcium leucovorin for hepatocellular carcinoma: a phase II trial. Cancer Invest. 1995; 13:460–463. PMID:
7552810.

627. Yang TS, Lin YC, Chen JS, Wang HM, Wang CH. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2000; 89:750–756. PMID:
10951336.

628. Guan Z, Wang Y, Maoleekoonpairoj S, et al. Prospective randomised phase II study of gemcitabine at standard or fixed dose rate schedule in unresectable hepatocellular carcinoma. Br J Cancer. 2003; 89:1865–1869. PMID:
14612894.

629. Yen Y, Lim DW, Chung V, et al. Phase II study of oxaliplatin in patients with unresectable, metastatic, or recurrent hepatocellular cancer: a California Cancer Consortium Trial. Am J Clin Oncol. 2008; 31:317–322. PMID:
18845988.
630. Patt YZ, Hassan MM, Aguayo A, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004; 101:578–586. PMID:
15274071.

631. Boige V, Taieb J, Hebbar M, et al. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: a multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur J Cancer. 2006; 42:456–459. PMID:
16427779.

632. Yuen MF, Poon RT, Lai CL, et al. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology. 2002; 36:687–691. PMID:
12198662.

633. Barbare JC, Bouche O, Bonnetain F, et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: a phase III multicentre, randomised, double blind placebo-controlled study. Eur J Cancer. 2009; 45:1788–1797. PMID:
19303768.

634. Llovet JM, Sala M, Castells L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000; 31:54–58. PMID:
10613728.

635. Barbare JC, Bouche O, Bonnetain F, et al. Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol. 2005; 23:4338–4346. PMID:
15994145.

636. Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013; 31:3501–3508. PMID:
23980077.

637. Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014; 19:1169–1178. PMID:
25223462.

638. Zaanan A, Williet N, Hebbar M, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013; 58:81–88. PMID:
22989572.

639. Patrikidou A, Sinapi I, Regnault H, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Invest New Drugs. 2014; 32:1028–1035. PMID:
24748335.

640. Petrelli F, Coinu A, Borgonovo K, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma: a systematic review and pooled analysis. Clin Oncol (R Coll Radiol). 2014; 26:488–496. PMID:
24856442.

641. Chiu CH, Liu YH, Wang YC, et al. In vitro activity of SecA inhibitors in combination with carbapenems against carbapenem-hydrolysing class D beta-lactamase-producing Acinetobacter baumannii. J Antimicrob Chemother. 2016; 71:3441–3448. PMID:
27543656.
642. Thomas MB. Systemic therapy for hepatocellular carcinoma. Cancer J. 2008; 14:123–127. PMID:
18391618.

643. Lim TY, Cheong JY, Cho SW, et al. Effect of low dose 5-fluorouracil and cisplatin intra-arterial infusion chemotherapy in advanced hepatocellular carcinoma with decompensated cirrhosis. Korean J Hepatol. 2006; 12:65–73. PMID:
16565607.
644. Woo HY, Bae SH, Park JY, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010; 65:373–382. PMID:
19763572.

645. Hamada A, Yamakado K, Nakatsuka A, Takaki H, Akeboshi M, Takeda K. Hepatic arterial infusion chemotherapy with use of an implanted port system in patients with advanced hepatocellular carcinoma: prognostic factors. J Vasc Interv Radiol. 2004; 15:835–841. PMID:
15297587.

646. Ueshima K, Kudo M, Takita M, et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010; 78(Suppl 1):148–153. PMID:
20616598.

647. Kudo M, Izumi N, Sakamoto M, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016; 5:190–197. PMID:
27493894.

648. Terashima T, Yamashita T, Arai K, et al. Beneficial effect of maintaining hepatic reserve during chemotherapy on the outcomes of patients with hepatocellular carcinoma. Liver Cancer. 2017; 6:236–249. PMID:
28626734.

649. Kawaoka T, Aikata H, Hyogo H, et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis. 2015; 16:505–512. PMID:
26121102.
650. Jeong SW, Jang JY, Lee JE, et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol. 2012; 8:164–171. PMID:
22524575.

651. Song DS, Song MJ, Bae SH, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015; 50:445–454. PMID:
25027973.

652. Fukubayashi K, Tanaka M, Izumi K, et al. Evaluation of sorafenib treatment and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a comparative study using the propensity score matching method. Cancer Med. 2015; 4:1214–1223. PMID:
26044168.

653. Ikeda M, Shimizu S, Sato T, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016; 27:2090–2096. PMID:
27573564.

654. Kudo M, Ueshima K, Yokosuka O, et al. Prospective randomized controlled phase III trial comparing the efficacy of sorafenib versus sorafenib in combination with low-dose cisplatin/fluorouracil hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Hepatol. 2016; 64(2 Suppl):S209–S210.

655. Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004; 15:1661–1666. PMID:
15520068.

656. Nagamatsu H, Itano S, Nagaoka S, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004; 99:2369–2375. PMID:
15571585.

657. Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007; 136:699–712. PMID:
17338776.

658. Mindikoglu AL, Regev A, Schiff ER. Hepatitis B virus reactivation after cytotoxic chemotherapy: the disease and its prevention. Clin Gastroenterol Hepatol. 2006; 4:1076–1081. PMID:
16861051.

659. Lau GK, He ML, Fong DY, et al. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology. 2002; 36:702–709. PMID:
12198664.

660. Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000; 62:299–307. PMID:
11055239.

661. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007; 45:507–539. PMID:
17256718.

662. Wu XY, Li X, Chen ZH, et al. An optimized antiviral modification strategy for prevention of hepatitis B reactivation in patients undergoing prophylactic lamivudine and chemotherapy: a pilot study. Tumour Biol. 2013; 34:909–918. PMID:
23269606.

663. Cortelezzi A, Vigano M, Zilioli VR, et al. Adefovir added to lamivudine for hepatitis B recurrent infection in refractory B-cell chronic lymphocytic leukemia on prolonged therapy with Campath-1H. J Clin Virol. 2006; 35:467–469. PMID:
16316778.

664. Huang L, Li J, Yan J, et al. Antiviral therapy decreases viral reactivation in patients with hepatitis B virus-related hepatocellular carcinoma undergoing hepatectomy: a randomized controlled trial. J Viral Hepat. 2013; 20:336–342. PMID:
23565616.

665. Lao XM, Luo G, Ye LT, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013; 33:595–604. PMID:
23402625.

666. Lao XM, Wang D, Shi M, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2011; 41:553–563. PMID:
21615643.

667. Firpi RJ, Nelson DR. Management of viral hepatitis in hematologic malignancies. Blood Rev. 2008; 22:117–126. PMID:
18343002.

668. Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006; 43:233–240. PMID:
16440357.

669. Park JW, Park KW, Cho SH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol. 2005; 100:2194–2200. PMID:
16181368.

670. Jang JW, Kwon JH, You CR, et al. Risk of HBV reactivation ac cording to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther. 2011; 16:969–977. PMID:
22024512.
671. Tamori A, Nishiguchi S, Tanaka M, et al. Lamivudine therapy for hepatitis B virus reactivation in a patient receiving intra-arterial chemotherapy for advanced hepatocellular carcinoma. Hepatol Res. 2003; 26:77–80. PMID:
12787809.

672. Nagamatsu H, Kumashiro R, Itano S, Matsugaki S, Sata M. Investigation of associating factors in exacerbation of liver damage after chemotherapy in patients with HBV-related HCC. Hepatol Res. 2003; 26:293–301. PMID:
12963429.

673. Kubo S, Nishiguchi S, Hamba H, et al. Reactivation of viral replication after liver resection in patients infected with hepatitis B virus. Ann Surg. 2001; 233:139–145. PMID:
11141236.

674. Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg. 2015; 261:56–66. PMID:
25072444.
675. Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007; 69:813–819. PMID:
17524569.

676. Dan JQ, Zhang YJ, Huang JT, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013; 39:865–872. PMID:
23597497.

677. Yoshida H, Yoshida H, Goto E, et al. Safety and efficacy of lamivudine after radiofrequency ablation in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Int. 2008; 2:89–94. PMID:
19669283.

678. Suh SJ, Yim HJ, Seo JH, et al. The risk of hepatitis B virus reactivation is considerably high during sorafenib therapy in patients with advanced hepatocellular carcinoma. J Hepatol. 2017; 66(1 Suppl):S449–S450.

679. Lin CL, Kao JH. Review article: novel therapies for hepatitis B virus cure - advances and perspectives. Aliment Pharmacol Ther. 2016; 44:213–222. PMID:
27302653.

680. Sung PS, Bae SH, Jang JW, et al. Differences in the patterns and outcomes of enhanced viral replication between hepatitis C virus and hepatitis B virus in patients with hepatocellular carcinoma during transarterial chemolipiodolization. Korean J Hepatol. 2011; 17:299–306. PMID:
22310794.

681. Hong SH, Roh SY, Kim SY, et al. Change in cancer pain management in Korea between 2001 and 2006: results of two nationwide surveys. J Pain Symptom Manage. 2011; 41:93–103. PMID:
20870388.

682. Kim JY, Jang WY, Hur MH, et al. Prevalence and management of pain by different age groups of Korean cancer patients. Am J Hosp Palliat Care. 2013; 30:393–398. PMID:
22777412.

683. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007; 18:1437–1449. PMID:
17355955.
684. Grudzen CR, Richardson LD, Johnson PN, et al. Emergency department-initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol. 2016; 2:591–598. PMID:
26768772.
685. Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015; 33:1438–1445. PMID:
25800768.

686. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014; 383:1721–1730. PMID:
24559581.

687. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010; 363:733–742. PMID:
20818875.

688. Carr BI, Pujol L. Pain at presentation and survival in hepatocellular carcinoma. J Pain. 2010; 11:988–993. PMID:
20418170.

689. Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs. 2010; 33:3–10. PMID:
19926981.

690. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008; 64:1147–1161. PMID:
18762933.

691. Radner H, Ramiro S, Buchbinder R, Landewé RB, van der Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012; 1:CD008951. PMID:
22258995.
692. World Health Organization (WHO). Cancer pain relief: with a guide to opioid availability. Geneva: WHO;1996.
693. Ministry of Health & Welfare. Cancer pain management guideline. Seoul: Ministry of Health & Welfare;2012.
694. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guideline in oncology: adult cancer pain. Vol. 1. Fort Washington, MD: NCCN;2013.
695. Rossi S, Assis DN, Awsare M, et al. Use of over-the-counter analgesics in patients with chronic liver disease: physicians' recommendations. Drug Saf. 2008; 31:261–270. PMID:
18302450.
696. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005; 42:1364–1372. PMID:
16317692.

697. U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Drugs: acetaminophen information. Silver Spring, MD: U.S. Department of Health and Human Services;2013.
698. U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Drugs: acetaminophen information. Silver Spring, MD: U.S. Department of Health and Human Services;2017.
699. Mofredj A, Cadranel JF, Darchy B, et al. Hepatotoxicity caused by therapeutic doses of paracetamol in alcoholics: report of 2 cases of fatal hepatitis in cirrhosis. Ann Med Interne (Paris). 1999; 150:507–511. PMID:
10615538.
700. Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007; 27:1219–1230. PMID:
17723075.

701. Kuffner EK, Green JL, Bogdan GM, et al. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients: a multicenter randomized study. BMC Med. 2007; 5:13. PMID:
17537264.

702. Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007; 26:283–290. PMID:
17593074.
703. Khalid SK, Lane J, Navarro V, Garcia-Tsao G. Use of over-the-counter analgesics is not associated with acute decompensation in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009; 7:994–999. PMID:
19394441.

704. Villeneuve JP, Raymond G, Bruneau J, Colpron L, Pomier-Layrargues G. Pharmacokinetics and metabolism of acetaminophen in normal, alcoholic and cirrhotic subjects. Gastroenterol Clin Biol. 1983; 7:898–902. PMID:
6653975.
705. Hirschfield GM, Kumagi T, Heathcote EJ. Preventative hepatology: minimising symptoms and optimising care. Liver Int. 2008; 28:922–934. PMID:
18783540.

706. Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005; 12:133–141. PMID:
15767831.

707. Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010; 85:451–458. PMID:
20357277.

708. Williams RL, Upton RA, Cello JP, et al. Naproxen disposition in patients with alcoholic cirrhosis. Eur J Clin Pharmacol. 1984; 27:291–296. PMID:
6510456.

709. Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol. 2010; 16:5651–5661. PMID:
21128314.

710. Riley TR 3rd, Smith JP. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol. 1998; 93:1563–1565. PMID:
9732947.

711. Ackerman Z, Cominelli F, Reynolds TB. Effect of misoprostol on ibuprofen-induced renal dysfunction in patients with decompensated cirrhosis: results of a double-blind placebo-controlled parallel group study. Am J Gastroenterol. 2002; 97:2033–2039. PMID:
12190173.

712. Castro-Fernandez M, Sanchez-Munoz D, Galan-Jurado MV, et al. Influence of nonsteroidal antiinflammatory drugs in gastrointestinal bleeding due to gastroduodenal ulcers or erosions in patients with liver cirrhosis. Gastroenterol Hepatol. 2006; 29:11–14. PMID:
16393624.
713. Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non-steroidal anti-inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int. 2012; 32:859–866. PMID:
22226322.

714. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009; 84:613–624. PMID:
19567715.

715. Hasselström J, Eriksson S, Persson A, Rane A, Svensson JO, Säwe J. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol. 1990; 29:289–297. PMID:
2310653.
716. Tegeder I, Lotsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999; 37:17–40. PMID:
10451781.

717. Kotb HI, El-Kady SA, Emara SE, Fouad EA, El-Kabsh MY. Pharmacokinetics of controlled release morphine (MST) in patients with liver carcinoma. Br J Anaesth. 2005; 94:95–99. PMID:
15516353.

718. Kotb HI, Fouad IA, Fares KM, Mostafa MG, Abd El-Rahman AM. Pharmacokinetics of oral tramadol in patients with liver cancer. J Opioid Manag. 2008; 4:99–104. PMID:
18557166.
719. Tallgren M, Olkkola KT, Seppälä T, Hkerstedt K, Lindgren L. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther. 1997; 61:655–661. PMID:
9209248.

720. Durnin C, Hind ID, Ghani SP, Yates DB, Molz KH. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in subjects with moderate hepatic impairment. Proc West Pharmacol Soc. 2001; 44:83–84. PMID:
11794005.
721. Haberer JP, Schoeffler P, Couderc E, Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth. 1982; 54:1267–1270. PMID:
7171414.

722. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID:
7459811.

723. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID:
10655437.
724. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID:
19097774.

725. Facciuto ME, Rochon C, Pandey M, et al. Surgical dilemma: liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis: intention-to-treat analysis in patients within and outwith Milan criteria. HPB (Oxford). 2009; 11:398–404. PMID:
19768144.

726. Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009; 115:616–623. PMID:
19117042.
727. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001; 35:421–430. PMID:
11592607.
728. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60. PMID:
20175033.

729. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017; 18:e143–e152. PMID:
28271869.

730. Yamamoto K, Imamura H, Matsuyama Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010; 45:1272–1282. PMID:
20625772.

731. Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009; 16:792–794. PMID:
19190964.

732. Liu D, Chan AC, Fong DY, Lo CM, Khong PL. Evidence-based surveillance imaging schedule after liver transplantation for hepatocellular carcinoma recurrence. Transplantation. 2017; 101:107–111. PMID:
28009758.

733. Hyder O, Dodson RM, Weiss M, et al. Trends and patterns of utilization in post-treatment surveillance imaging among patients treated for hepatocellular carcinoma. J Gastrointest Surg. 2013; 17:1774–1783. PMID:
23943387.

734. Zheng J, Chou JF, Gonen M, et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017; 266:693–701. PMID:
28650354.
735. Dioguardi Burgio M, Ronot M, Fuks D, et al. Follow-up imaging after liver transplantation should take into consideration primary hepatocellular carcinoma characteristics. Transplantation. 2015; 99:1613–1618. PMID:
25710611.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


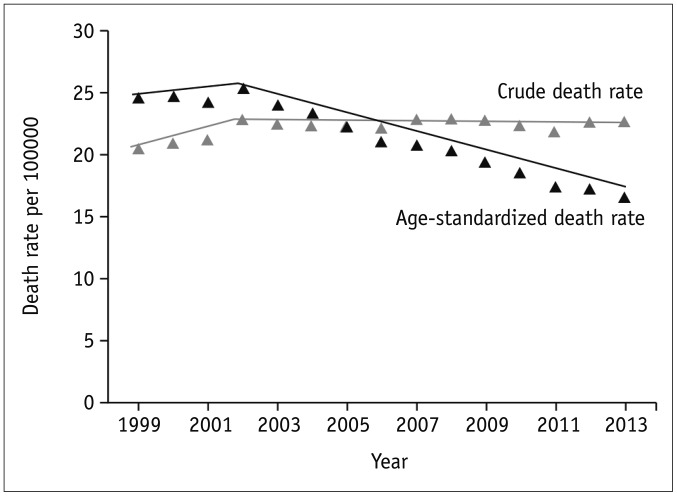

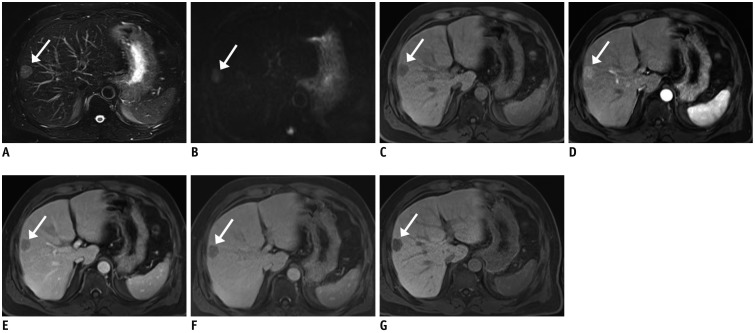
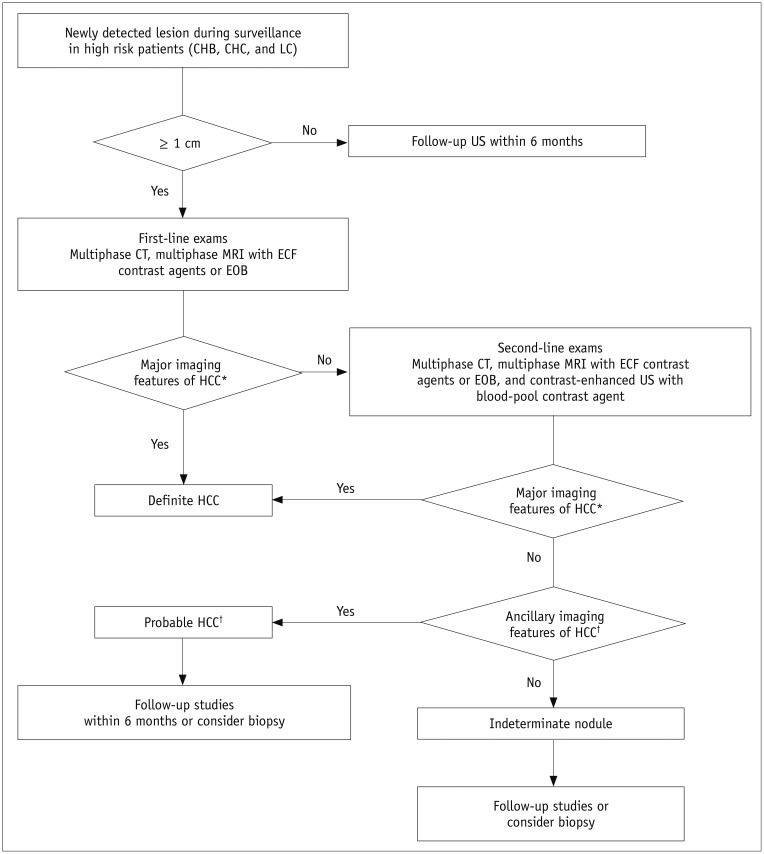
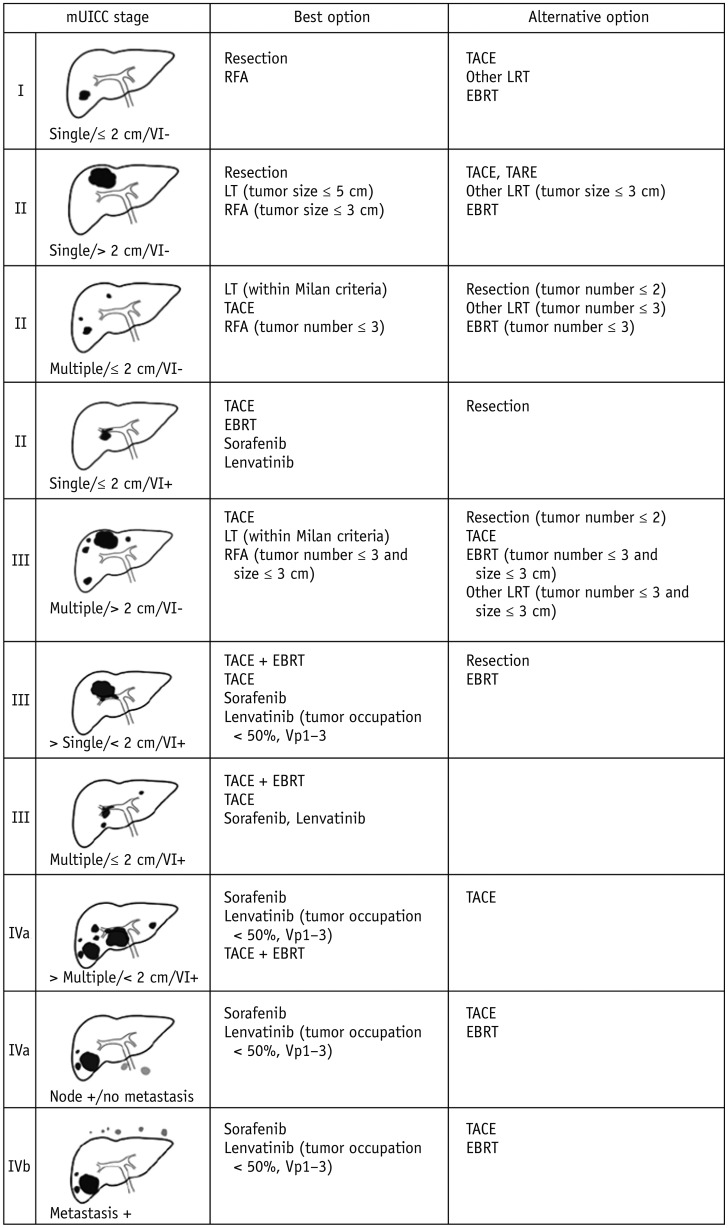
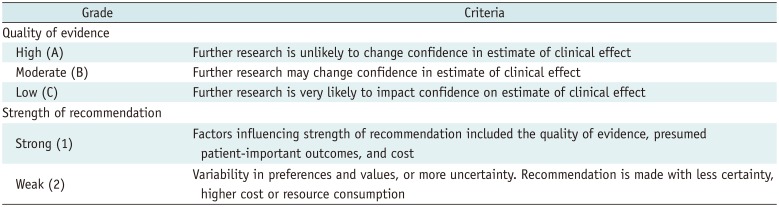


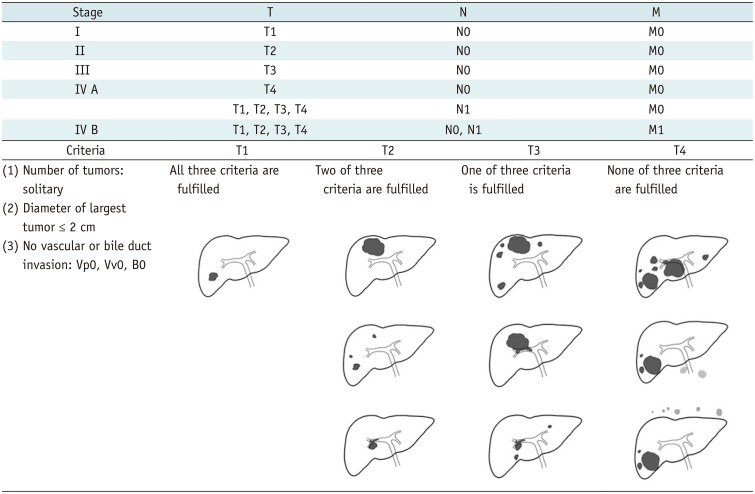
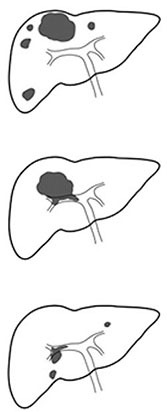
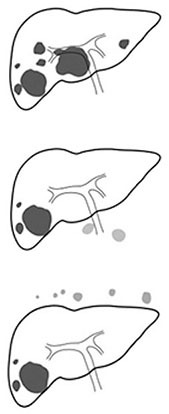
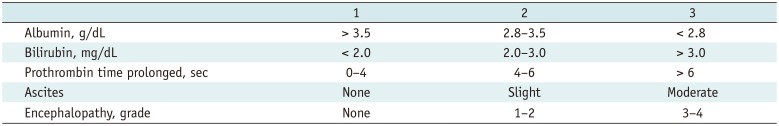
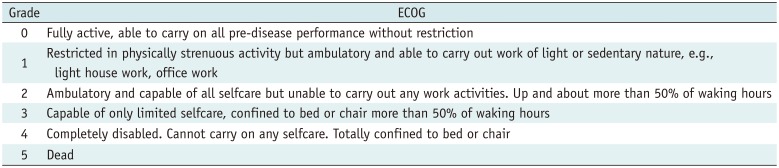
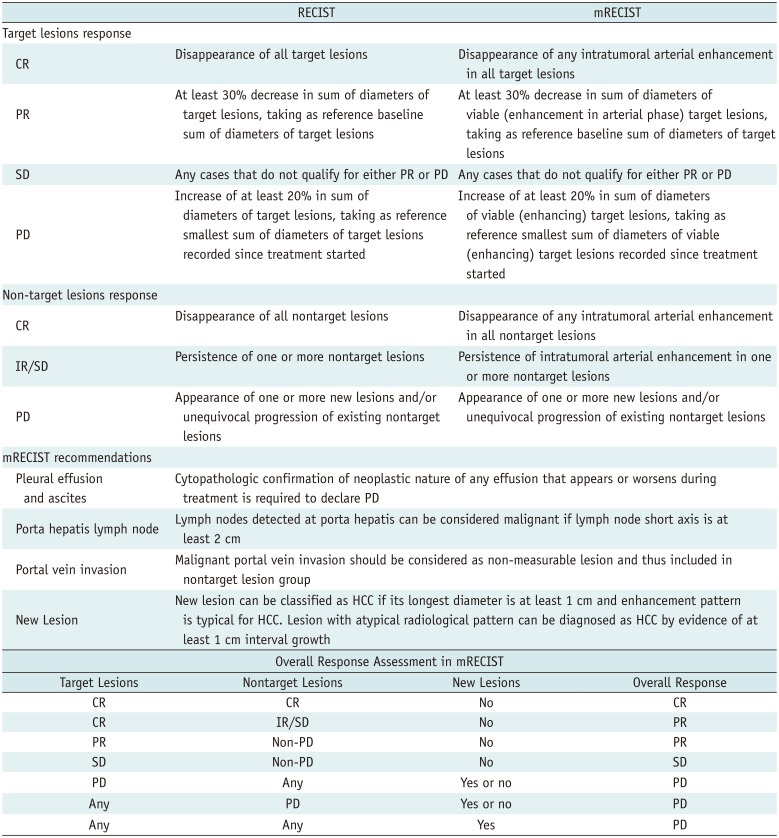
 XML Download
XML Download