Abstract
Purpose
A dynamic thiol/disulfide balance is pivotal in organizing anti-oxidant defense, detoxification, apoptosis, and enzyme activities, as well as transcription and cellular signal-transfer mechanisms. The connection between urolithiasis and oxidant/antioxidant status, which can be assessed through thiol-disulfide homeostasis (TDH), has not yet been examined. In this study, we evaluated the effects of TDH on the formation, size, and location of stones by examining the associations between TDH parameters and urolithiasis.
Materials and Methods
Patients with urolithiasis and healthy controls were recruited. The patients were divided into subgroups in terms of stone size (>15 mm or ≤15 mm) and stone location (nephrolithiasis or ureterolithiasis). TDH parameters were measured using a novel automatic and spectrophotometric method and compared statistically.
Results
TDH parameters were different between the urolithiasis and control groups. TDH tended towards the disulfide side in the urolithiasis group. Stone size increased an average 0.14 mm with a 1 µmol/L increase in disulfide level and decreased an average 0.058 mm with a 1 µmol/L increase in native thiol level. Disulfide and native thiol levels were found to be different across patients with stone size >15 mm, ≤15 mm, and controls (p<0.001 and p<0.001, respectively). However, the nephrolithiasis and ureterolithiasis groups were similar in respect of TDH parameters.
Urolithiasis, also known as urinary system stone disease, is a health problem that affects populations worldwide. The prevalence rates for urolithiasis range between 1% and 20%. Urolithiasis is a multi-factorial disease, environmental factors such as lifestyle, obesity, nutrition habits, and dehydration are drivers of urolithiasis, and hormonal, genetic or anatomic factors affect its pathogenesis [1234].
Although significant improvements have been made in both the recognition and management of the disease in recent years, contributing factors to the etiology of urolithiasis are still being investigated. In previous studies, it was found that markers for oxidative stress increased in patients with urolithiasis compared with healthy people [56].
It has been shown in recent studies that increased reactive oxygen species (ROS) and lipid peroxidation plays a role in the pathogenesis of patients with urolithiasis [56789]. Thiol is an organic compound that contains a sulfhydryl (-SH) group, which has a critical role in preventing the formation of any oxidative stress condition in cells. Thiol groups are important members of the antioxidant cascade. Their -SH groups react with reactive oxidant molecules and degrade them, thereby protecting the organism from oxidative damage [1011]. The oxidation reaction of thiols with oxidizing molecules causes the formation of reversible disulfide bonds. This redox is the earliest observable symptom of radical-mediated protein oxidation. The disulfide bond structures formed when the oxidative stress condition ends can be reduced to thiol groups again and thus the dynamic thiol/disulfide balance is preserved. Evaluation of this balance provides information about the oxidative status in plasma. A dynamic thiol/disulfide balance is pivotal in organizing anti-oxidant defense, detoxification, apoptosis, and enzyme activities, as well as transcription and cellular signal-transfer mechanisms [101112]. A disturbed thiol/disulfide balance is observed in many diseases such as diabetes mellitus, cardiovascular diseases, cancer, and kidney failure [8131415].
The connection between urolithiasis and oxidant/antioxidant status, which can be assessed through thiol-disulfide homeostasis (TDH), has not yet been examined. In this study, we evaluated the effects of TDH on the formation, size, and location of stones by examining the associations between TDH parameters and urolithiasis.
The article is in accordance with ethical standards and was approved by Necmettin Erbakan University Meram Faculty of Medicine Ethics Committee (approval number: 2017/959). Informed consent was obtained from all individual participants included in the study.
Patients diagnosed with nephrolithiasis or ureterolithiasis after radiologic evaluation of computed tomography (CT) scans were recruited for the study. Patients who were recently diagnosed as having stones, aged >18 years, with no previous surgical stone treatment and extracorporeal shock wave lithotripsy (ESWL), were enrolled. The exclusion criteria included the presence of any systemic, chronic and malignant diseases, smoking or alcohol consumption, presence of urinary or systemic infection, and intake of any dietary supplements or medications that could affect serum concentrations of TDH parameters.
Healthy individuals with similar demographic characteristics to the study group who had no past diagnoses of urolithiasis, cigarette and alcohol use, and chronic disease that could affect TDH, were recruited for the control group.
The study consisted of 76 patients with urolithiasis and 50 healthy controls. The patients with urolithiasis were also divided into subgroups according to both stone size (larger than 15 mm and smaller than 15 mm), location of the stone (nephrolithiasis and ureterolithiasis) and presence of renal colic
Data such as number, size, surface area, density, side, and hydronephrosis status of stones were recorded according to CT reports. Age, body mass index, biochemical and hematologic values of all patients were recorded.
Peripheral venous bloodsamples (5 mL) were drawn into serum separator tubes (Vacuette; Greiner Bio-One, Kremsmuenster, Austria) after overnight fasting. Sera samples were left 30 to 60 minutes to form clots prior to centrifugation at 1,500g for 10 minutes at room temperature and the sera were then stored at −80℃ until required for analysis.
Thiol/disulfide homeostasis parameters were measured using a novel automatic and spectrophotometric method [12]. Free functional thiol groups (-SH) were extricated by decreasing disulfide bonds (-S-S-) using sodium borohydride (NaBH4). Unused NaBH4 remnants were completely removed using formaldehyde. This prevented further reduction of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) as well as any disulfide bonds resulting from possible reactions with DTNB. Total thiol groups including reduced and native thiol groups were determined after reactions with DTNB. The disulfide parameter value can be calculated as half of the native thiol content and total thiol content. Disulfide/total thiol, disulfide/native thiol, and native thiol/total thiol ratios were calculated.
Routine biochemical, hematologic and urine analysis results were obtained by reviewing the patients' records. Hematologic analyses were performed using an XN-1000 Sysmex (Sysmex Corporation, Kobe, Japan) hematology analyzer. All biochemical parameters were analyzed with using Abbott Kits (Abbott Laboratories, Chicago, IL, USA), which are manufactured for use with an Architect c16000 Auto-Analyzer. Urine samples were analyzed using a Dirui FUS-200 (Dirui Industrial Co. Ltd., Changchun, China) automatic urine sediment analyzer.
Stone analysis was made using “Fourier-transform infrared spectroscopy” for patients who had undergone surgery due to urolithiasis.
Statistical analysis was performed using a SAS University Edition 9.4 (SAS Institute Inc.,Cary, NC, USA). Continuous variables are presented as mean and standard deviations. Categorical variables are given as frequencies and percentages. The independent samples t-test or ANOVA were used when comparing continuous variables among groups. Tukey and Games-Howell post-hoc comparisons were performed after a significant ANOVA. Chi-square tests were used to compare two groups in terms of categorical variables. Pearson correlation coefficients of continuous variables were calculated. Simple linear regression models were formed to determine the effects of thiol/disulfide hemostasis parameters on stone size, surface area, and density. An ordinal logistic regression model is formed to find variables associated with group member ship. A p-value <0.05 is considered statistically significant.
A total of 76 patients and 50 healthy controls were enrolled in the study. There were 76 patients with nephrolithiasis and ureterolithiasis, of which 43 patients (56.6%) had nephrolithiasis and 33 patients (43.4%) had ureterolithiasis. Surgical treatment was performed in 66 (86.8%) patients with urolithiasis. CT scan data and stone analysis details of urolithiasis patients are presented in Table 1.
There was no significant difference between demographic data, biochemical parameters, and urine-related parameters amongst stone group (n=76) and stone free control group (n=50). Although the neutrophil count and C-reactive protein (CRP) level among systemic inflammatory markers were found to be high in the urolithiasis group (p=0.002 and p=0.001, respectively), no significant difference was detected in the white blood cell (WBC) count, lymphocyte count, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) between these groups. Statistically significant differences were detected in levels of native thiol, total thiol, disulfide, disulfide/total thiol, disulfide/native thiol, and native thiol/total thiol ratios (p<0.001, p<0.001, p<0.001, p<0.001, p<0.001, p<0.001, respectively). It was found that native thiol and total thiol levels were lower and that the disulfide level was higher in the urolithiasis group; thiol/disulfide homeostasis tended towards the disulfide side. No differences were detected between stone and control groups in terms of demographic, biochemical, and hematologic data (Table 2).
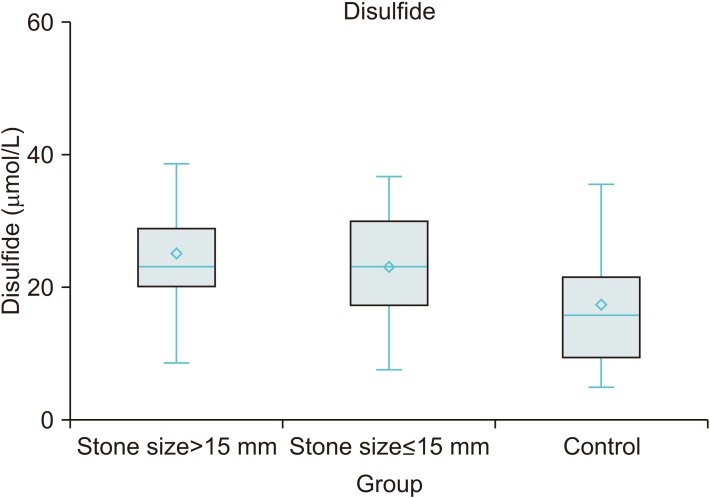
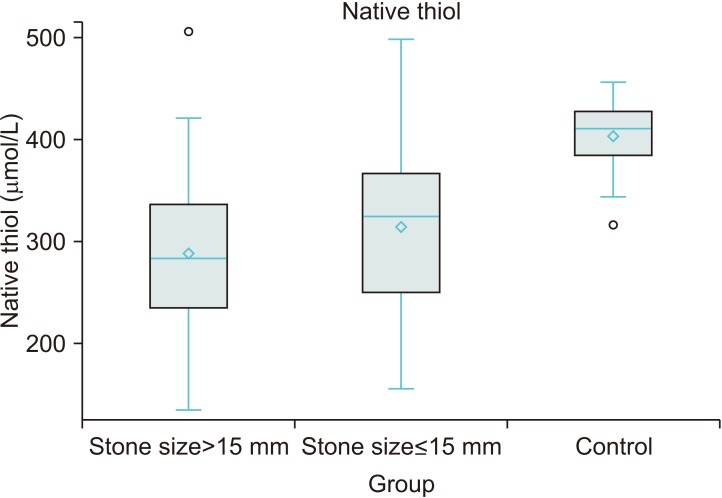
Although TDH tended towards the disulfide side in stone subgroups determined by stone size, TDH parameters were not found to be different between the subgroups (≤15 mm vs. >15 mm). Besides, there was no significant difference in the systemic inflammatory marker levels between these groups (Table 3). However, in the triple group analysis including >15 mm, ≤15 mm, and stone free control groups; disulfide and native thiol levels were found to be different across patients with stone size >15 mm, ≤15 mm, and controls (p<0.001 and p<0.001, respectively). Post-hoc comparisons showed that disulfide was higher and native thiol was lower in the stone group compared to those of the control group (p=0.001, p=0.015, p<0.001, and p<0.001, respectively) (Figs. 1, 2).
No significant correlations were detected between TDH parameters and stone size, surface area, and stone density. Simple regression analyses showed that stone size increased on average by 0.14 mm with a 1 µmol/L increase in disulfide, and decreased by 0.058 mm with a 1 µmol/L increase in native thiol.
A backward ordinal logistic regression model was carried out to find relevant variables with group membership (three levels: control and two stone subgroups). Native thiol and disulfide variables remained in the final step of the procedure and all other variables were excluded from the model. A unit increase in native thiol was found to be associated with a 1% decrease in the odds of being in a larger-sized stone group, and a unit increase in disulfide was associated with a 6% increase in the odds of being in a larger-sized stone group (p<0.0001 and p<0.004, respectively).
No significant differences were detected between patients with nephrolithiasis and ureterolithiasis in terms of TDH parameters and systemic inflammatory marker levels (Table 3). No significant associations were found between presence of hydronephrosis and TDH parameters (results not given).
Stone patients were also separated into two sub groups based on whether they are renal colic (n=37) or not (n=39). No significant difference was detected in systemic inflammatory parameters and TDH parameters among these groups (Table 4). A significant difference was also observed in all TDH parameters among stone patients who did not have renal colic and the stone free control group. Additionally, it was detected that TDH hemostasis had a tendency towards disulfide side (all parameters p<0.05).
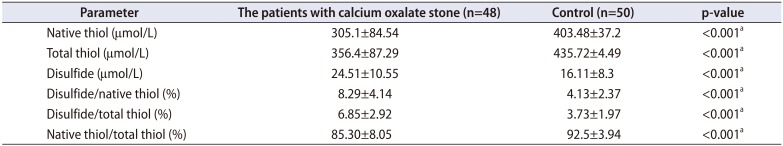
An additional analysis was performed comparing the TDH parameters in patients with calcium oxalate stones (n=48 patients) and healthy controls; these results are shown in Table 5.
The underlying etiologic factors of the stone formation are genetic, metabolic, anatomic, environmental, and dietary factors. Although urine crystals are continuously excreted through urine in physiologically, pathologic urolithiasis formmation may occur in a small number of people. In the event of renal tubular damage and the increase in urine supersaturation, the tendency towards urolithiasis increases. Although supersaturation is essential for crystal formation, it is not the only causal agent for stone formation [161718]. The crystal formation, mainly in the form of calcium phosphate and calcium oxalate crystals, is prevalent in urinary tracts. Continuous crystalluria is harmful to the kidney epithelium and it may result in ROS production, which may cause cellular damage and cellular death. The main responsible factors in oxidative damage process of biomolecules are ROS [1819]. The more exposure to extreme amounts of crystals in the urinary system, the more ROS production occurs which is tried to be compensated by endogen antioxidants. Nevertheless, cellular damage still occurs. Cellular damage supports stone formation through nucleation, aggregation, and crystal adherence on the kidney epithelium. All these mechanisms show that the inflammatory response and oxidative stress are underliying factors of stone formation [16171819].
Serum CRP is a nonspecific marker of systemic inflammation. CRP is an acute-phase protein whose blood levels increase in the presence of inflammation or tissue injury. CRP is present in plasma at a concentration <5 mg/L, and concentrations >5 mg/L show the presence of inflammation [20]. Although there were no significant differences in urine WBC levels between the urolithiasis group and the control group, a significant difference was found with CRP levels (7.03 vs. 2.95 mg/L, p=0.001). This condition suggests the presence of a considerable amount of inflammation without an infection process in patients with urolithiasis.
The NLR and PLR are inflammatory markers used as prognostic factors in various diseases [21]. In our study, although the neutrophil count (which supports inflammation) was higher than in the control group, NLR and PLR values were not significantly different. In patients with urolithiasis, the relationship between oxidative stress and inflammation is an attractive issue for study.
Additionally, no significant difference was observed in systemic inflammatory markers among stone size (≤15 mm vs. >15 mm), stone localization (nephrolithiasis vs. ureterolithiasis), presence of renal colic (renal colic+ vs. renal colic−) subgroups. CRP levels were over 5 mg/L in all subgroups (Tables 3, 4). These results make us consider that inflammation is directly related to stone presence itself, instead of stone size, stone localization and/or presence of renal colic.
The oxidant/antioxidant status of individuals depends on many factors [16]. The imbalance between oxidant and antioxidant systems is defined as oxidative stress. Although ROS play a significant role in cellular signal transfer in small concentrations, they may cause irreversible damage to cellular macromolecules such as lipids, proteins, and DNA when they are produced in large amounts or cannot be eliminated by antioxidants [9].
Microstructural changes of renal tubular cells in the early phases of urinary stone formation were examined in some previous studies. It was reported that oxidative stress related to mitochondrial deformation played a certain role in urinary crystal formation, and immuno-transmission electron microscopy showed the presence of mitochondrial damage in crystal nuclei [192223].
The relationship between stone formation and oxidative stress has been examined in a limited number of studies. Grases et al. [24] reported that oxidative stress played a role in renal stones containing calcium oxalate monohydrate. Ma et al. [6] stated that erythrocyte oxidative stress caused renal tubular damage and stone formation in patients with hyperoxaluria. Göknar et al. [16] reported that oxidative stress was central to stone formation in pediatric patients. It was also reported that antioxidant materials such as green tea, vitamin E, and superoxide dismutase decreased crystal formation and cellular damage causing stone formation [2526].
Sulfur-containing compounds called thiol groups constitute a key part of the antioxidant cascade, and these are important compounds that eliminate free radicals through enzymatic or non-enzymatic pathways [27]. Thiol groups form a disulfide compound through oxidation with ROS. The transformation of thiols into disulfide is the earliest indicator of ROS-mediated protein oxidation. Thiol groups reversibly transformed into disulfide structures may cause a decrease in thiol concentrations. Thus, TDH is preserved [92728]. TDH plays a key role in detoxification, signal transfer, enzyme activity regulation, and apoptosis [2728].
A number of studies have revealed that in degenerative diseases such as diabetes, obesity, and pneumonia, TDH tended towards the disulfide side, whereas in some proliferative diseases such as psoriasis and cancer, it tended towards the native thiol side. It was also claimed that an increase in disulfide level was an indicator of oxidative stress, whereas an increase in native thiol was an indicator of anti-oxidant response to ROS [12131427282930].
The role of oxidative stress in urolithiasis and its effect on TDH has not yet been explored.
In this study, it was observed that in urolithiasis patients, there was a tendency towards disulfide side of TDH and their thiol levels were significantly lower than stone free control group. Additionally, patients with a stone size larger than 15 mm had higher disulfide levels and lower thiol levels than patients with a stone size lower than 15 mm, but this difference was not statistically significant. Ma et al. [6] reported that erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and also renal tubular damage.
This study showed that TDH tendency towards the disulfide side in patients with urolithiasis may indicate oxidative stress compared to stone free patients. According to the previous studies native thiol is the antioxidant indicator though disülfide is the oxidative stress indicator. Therefore, our study has shown inadequate antioxidant response and increased oxidative stress occurs.
Native thiol levels were lower and the disulfide levels were higher in patients with stone size greater than 15 mm. Although these values are not significant, we think that it may contribute to the relationship between oxidative stress, antioxidant response and presence of stones. Because 1 unit of disulfide increase in logistic regression analysis also covering stone free control group increases the possibility of a bigger stone and vice versa for native thiol.
No difference was detected in TDH parameters among nephrolithiasis and ureterolithiasis groups in this study. Most of the ureteral stones occur due to replacement of kidney stones to ureter. So, the lack of any difference in oxidative stress parameters among these groups may be related to the fact that the stone was formed in the same place.
Although it appears that TDH parameters seem to have a tendency towards disulfide side in renal colic patients compared to non-renal colic patients, this was not statistically significant. Additionally, systemic inflammatory markers were also higher in renal colic patients although this was not significant too (Table 4). We think that this situation is related to the partial increase in inflammatory and oxidative stress markers due to pain. There was a significant difference in TDH parameters among non-renal colic stone patients (asymptomatic) and stone free control group in this study. It was measured that TDH hemostasis had a tendency towards disulfide side. These results indicate that there is a significant connection between stone presence and oxidative stress even though the patients are asymptomatic.
As a result, no significant difference was detected in TDH and inflammatory markers in all of the subgroups (stone localization, stone size, renal colic). Especially the significance in TDH parameters was significant among the overall patient group with urolihtiasis and stone free control groups.
Calcium oxalate stones are the most prevalent type; therefore, an additional analysis was performed comparing the TDH parameters only in patients with calcium oxalate stones and healthy controls. The results showed that TDH tended toward the disulphide side in patients with calcium oxalate stones. Oxalate is a toxic material and adheres to cell membranes, enhancing ROS release and lipid peroxidation, leading to cell necrosis and apoptosis. Therefore, an increase in oxalate in the body may be the main reason for the impairment in the antioxidant defense system and TDH.
Absence of TDH status after stone therapy and the relatively small number of patients can be listed as limitations of this study.
It was found that patients with urolithiasis displayed oxidative stress characterized by a TDH tendency towards the disulfide side, and an inadequate antioxidant response identified by a lower level of native thiol as compared with healthy controls. We propose that further studies be conducted that include post-treatment measurements of TDH parameters, along with a larger number of subgroups of different stone sizes and stone types.
References
1. Türk C, Neisius A, Petrik A, Seitz C, Skolarikos A, Tepeler A, et al. European Association of Urology guidelines on urolithiasis [Internet]. Arnhem: The European Association of Urology;2017. 3. cited 2019 Jan. Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis_2017_10-05V2.pdf.
2. Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, et al. Pathophysiology-based treatment of urolithiasis. Int J Urol. 2017; 24:32–38. PMID: 27539983.

3. Kaya E, Ebiloğlu T, Zor M, Yalççn S, Coğuplugil AE, Bedir S. The outcome of percutaneous nephrolithotomy on ≥50 mm staghorn and multiple calyceal stones. Turk J Urol. 2018; 44:148–152. PMID: 29511585.

4. Noureldin YA, da Silva A, Fahmy N, Andonian S. Is it safe to prescribe ascorbic acid for urinary acidification in stone-forming patients with alkaline urine? Turk J Urol. 2017; 43:183–188. PMID: 28717544.

5. Ceban E, Banov P, Galescu A, Botnari V. Oxidative stress and antioxidant status in patients with complicated urolithiasis. J Med Life. 2016; 9:259–262. PMID: 27974930.
6. Ma MC, Chen YS, Huang HS. Erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and renal tubular damage. Urology. 2014; 83:510.e9–510.e17.

7. Engin A, Altan N. Effects of obstructive jaundice on the antioxidative capacity of human red blood cells. Haematologia (Budap). 2000; 30:91–96. PMID: 10839561.

8. Yardim-Akaydin S, Sepici A, Ozkan Y, Torun M, Simşek B, Sepici V. Oxidation of uric acid in rheumatoid arthritis: is allantoin a marker of oxidative stress. Free Radic Res. 2004; 38:623–628. PMID: 15346653.

9. Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001; 477:7–21. PMID: 11376682.

10. Cremers CM, Jakob U. Oxidant sensing by reversible disulfide bond formation. J Biol Chem. 2013; 288:26489–26496. PMID: 23861395.

11. Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000; 72(2 Suppl):653S–669S. PMID: 10919972.

12. Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014; 47:326–332. PMID: 25304913.

13. Altıparmak IH, Erkuş ME, Sezen H, Demirbag R, Gunebakmaz O, Kaya Z, et al. The relation of serum thiol levels and thiol/disulphide homeostasis with the severity of coronary artery disease. Kardiol Pol. 2016; 74:1346–1353. PMID: 27221962.

14. Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel Ö, et al. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine. 2016; 51:47–51. PMID: 26547218.

15. Dirican N, Dirican A, Sen O, Aynali A, Atalay S, Bircan HA, et al. Thiol/disulfide homeostasis: a prognostic biomarker for patients with advanced non-small cell lung cancer? Redox Rep. 2016; 21:197–203. PMID: 26200761.

16. Göknar N, Oktem F, Arı E, Demir AD, Torun E. Is oxidative stress related to childhood urolithiasis? Pediatr Nephrol. 2014; 29:1381–1386. PMID: 24526098.

17. Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res. 2006; 34:86–91. PMID: 16404622.

18. Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012; 40:95–112. PMID: 22213019.

19. Fishman AI, Green D, Lynch A, Choudhury M, Eshghi M, Konno S. Preventive effect of specific antioxidant on oxidative renal cell injury associated with renal crystal formation. Urology. 2013; 82:489.e1–489.e7.

20. Yücel D. C-Reactive protein vs. high-sensitivity C-reactive protein: what is the difference? Turk J Biochem. 2014; 39:43–44.
21. Seropian IM, Romeo FJ, Pizarro R, Vulcano NO, Posatini RA, Marenchino RG, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of survival after heart transplantation. ESC Heart Fail. 2018; 5:149–156. PMID: 28758719.

22. Hirose M, Yasui T, Okada A, Hamamoto S, Shimizu H, Itoh Y, et al. Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int J Urol. 2010; 17:83–92. PMID: 19919640.

23. Hirose M, Tozawa K, Okada A, Hamamoto S, Higashibata Y, Gao B, et al. Role of osteopontin in early phase of renal crystal formation: immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol Res. 2012; 40:121–129. PMID: 21833789.

24. Grases F, Costa-Bauzá A, Bonarriba CR, Pieras EC, Fernández RA, Rodríguez A. On the origin of calcium oxalate monohydrate papillary renal stones. Urolithiasis. 2015; 43(Suppl 1):33–39. PMID: 25086903.

25. Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Preventive effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol. 2005; 173:271–275. PMID: 15592095.

26. Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urol Res. 2005; 33:261–266. PMID: 16047215.

27. Bal C, Büyükşekerci M, Koca C, AXğış ER, Erdoğan S, Baran P, et al. The compromise of dynamic disulfide/thiol homeostasis as a biomarker of oxidative stress in trichloroethylene exposure. Hum Exp Toxicol. 2016; 35:915–920. PMID: 26429930.

28. Emre S, Demirseren DD, Alisik M, Aktas A, Neselioglu S, Erel O. Dynamic thiol/disulfide homeostasis and effects of smoking on homeostasis parameters in patients with psoriasis. Cutan Ocul Toxicol. 2017; 36:393–396. PMID: 28397526.

29. Demirseren DD, Cicek C, Alisik M, Demirseren ME, Aktaş A, Erel O. Dynamic thiol/disulphide homeostasis in patients with basal cell carcinoma. Cutan Ocul Toxicol. 2017; 36:278–282. PMID: 28067074.

30. Hanikoglu F, Hanikoglu A, Kucuksayan E, Alisik M, Gocener AA, Erel O, et al. Dynamic thiol/disulphide homeostasis before and after radical prostatectomy in patients with prostate cancer. Free Radic Res. 2016; 50:S79–S84. PMID: 27620702.

Table 1
Computed tomography data and stone analysis details of the patients with urolithiasis
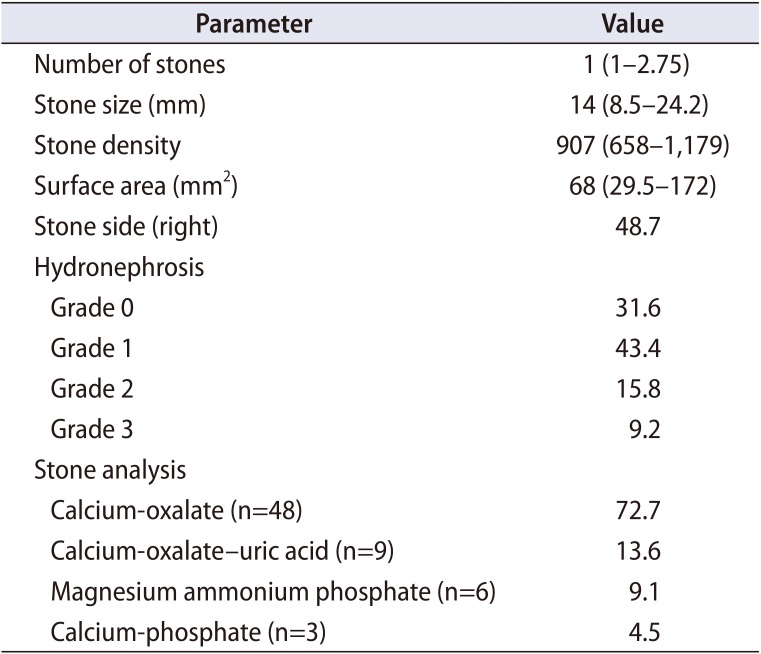
Table 2
Demographic variables, serum and urine parameters
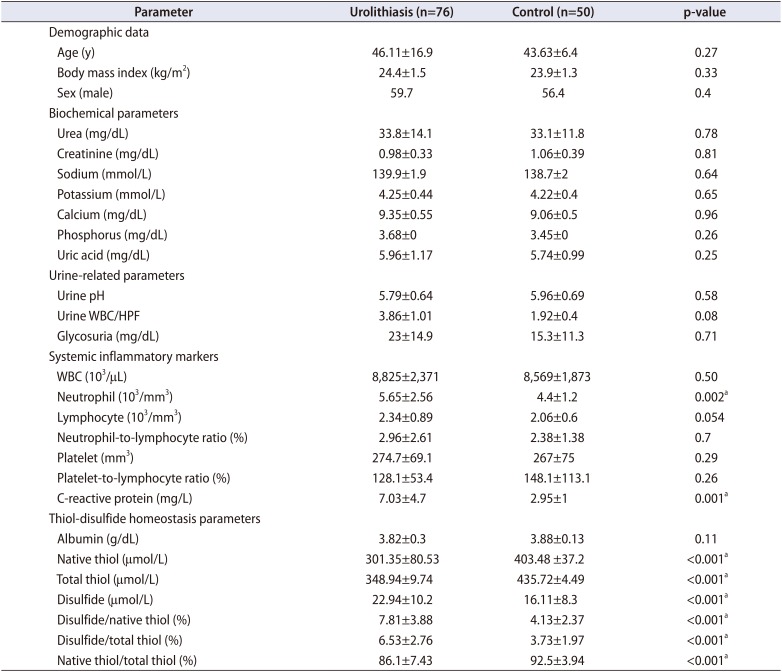
Table 3
Systemic inflammatory markers and TDH parameters for subgroups by stone size and location
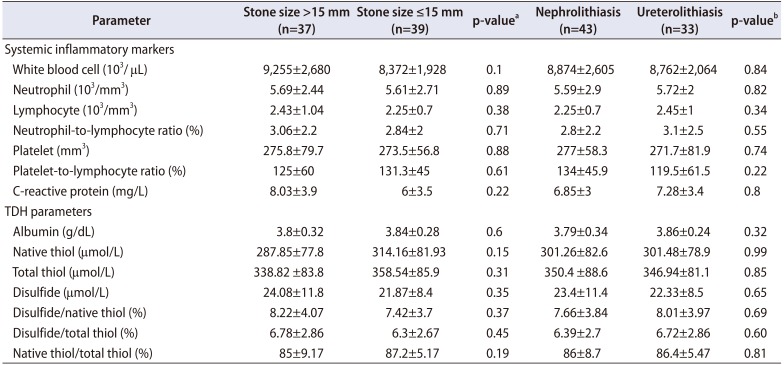
Table 4
TDH parameters and systemic inflammatory markers for subgroups with and without renal colic
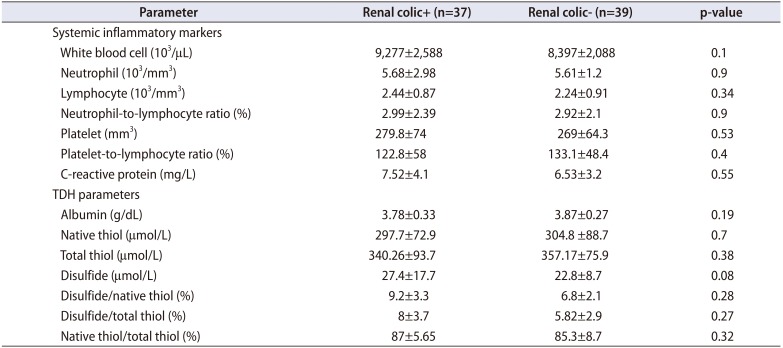
Table 5
Thiol-disulfide homeostasis parameters of patients with calcium oxalate stone and control group




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download