INTRODUCTION
The management of urolithiasis has been revolutionized with the introduction of shock wave lithotripsy (SWL) owing to its simplicity, noninvasive nature, efficacy, and minimal morbidity. Pain relief during SWL is vital, not only to maintain patient comfort and satisfaction, but also to facilitate stone imaging and targeting by reducing patient movement during successive shock wave impacts. Reduced patient movement increases the efficiency of fragmentation and reduces morbidity from SWL. The perception of pain during SWL is affected by patient-related factors like age, sex, and body habitus. Also, young female patients, anxious and depressed patients, and thin patients experience more pain during SWL [
123].
Analgesia for SWL includes intravenous (IV) propofol, extradural and epidural anesthetics, nitrous oxide, local anesthetic injection, and EMLA creams (a eutectic mixture of local anesthetics). Analgesics including paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids have also been found to provide sufficient pain control during SWL. Nontraditional methods such as music, acupuncture, and transcutaneous electrical nerve stimulation have also been used [
4].
Although European guidelines state that pain control during SWL is necessary, no recommendation is made for a specified analgesia, in contrast with a clear recommendation for pain relief for an acute renal pain episode [
5]. Consequently, 17 distinct analgesia regimens were used in 21 centers for SWL analgesia in the United Kingdom. More than one regimen was used in 50% of these centers. Diclofenac followed by pethidine and paracetamol were the most frequently used. IV fentanyl patient-controlled analgesia was used in 10.0% of the centers [
6].
In our SWL unit, pethidine is the standard analgesic used. We aimed to compare it with other opioid-sparing techniques, specifically, xylocaine gel and ketorolac, a potent analgesic with moderate anti-inflammatory activity with greater efficacy than that of morphine and pethidine [
7].
Go to :

MATERIALS AND METHODS
This was a single-blinded randomized controlled trial (RCT) and was registered at NIH
ClinicalTrials.gov (
http://www.clinicaltrials.gov/;
NCT03032458). Patients were aged ≥18 years with renal and upper ureteral stones amenable to SWL according to guidelines [
5] at the SWL unit at the Urology and Nephrology Center between January and October 2017. Allergy to pethidine, ketorolac, or xylocaine gel; an American Society of Anesthesiologists score ≥4; and body mass index ≥40 kg/m
2 were added as exclusion criteria [
5]. The CONSORT flowchart is shown in
Fig. 1.
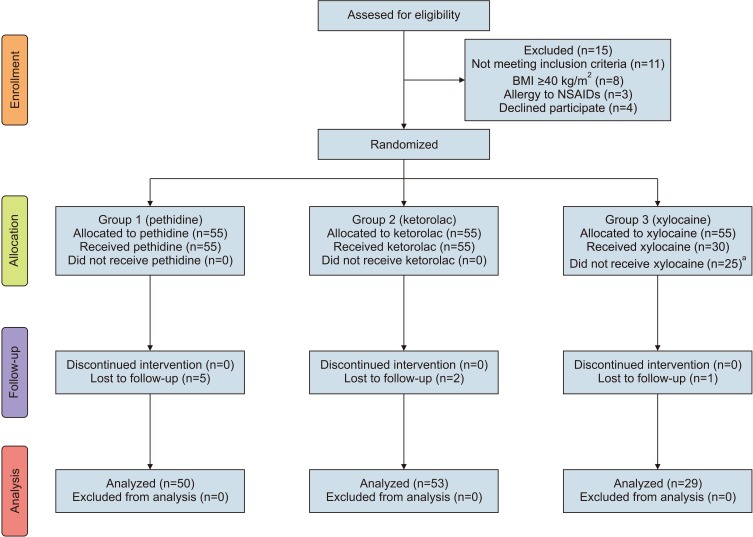 | Fig. 1CONSORT flowchart for study participants. BMI, body mass index; NSAIDs, nonsteroidal anti-inflammatory drugs. a:These patients received pethidine (our standard analgesia) and were excluded from our final analysis.
|
Patients were divided into three groups by use of computer-generated sequentially numbered randomization: the first group received pethidine (pethidine 25 mg/mL ampule (amp.) British Pharmacopoeia 2002; Misr, Cairo, Egypt) as an IV bolus injection before the session plus a placebo gel and then an IV infusion, so that the total dose did not exceed 1 mg/kg. The second group received an IV bolus injection of ketorolac (ketorolac 30 mg/2 mL amp., Amriya, Alexandria, Egypt) before the session plus a placebo gel and then a 30-mg IV infusion so that the total dose did not exceed 60 mg. The third group received a xylocaine gel (2% lidocaine gel, Astra Zeneca, Södertälje, Sweden) locally before the session with a 10-mg saline IV bolus before the session and then an IV saline infusion after. The drug doses were based on previous reviews and meta-analysis for postoperative pain control doses [
89]. The patients were blinded as to the used drug. An adjuvant anti-emetic was used if needed.
After the routine pre-procedural evaluation, SWL was performed using a Dornier lithotripter S (Dornier MedTech, Weßling, Germany) started at a power of 12 kV and then gradually increased to 17 kV. A total of 3,000 shocks was delivered per session or until complete fragmentation of the stone. The study primary outcome was to compare the efficacy of pethidine, ketorolac, and xylocaine gel in SWL analgesia. A validated 0 to 10 Numeric Pain Rating Scale (NPRS) [
10] was used for pain evaluation. The secondary outcome was to assess stone disintegration 2 weeks after the SWL session, SWL complications, and SWL session patientreported satisfaction between the groups. A single 4-tiered question regarding patient overall self-reported satisfaction about the efficacy of the analgesia efficacy was used (very satisfied, satisfied, unsatisfied, and very unsatisfied). Measurements of vital parameters during the SWL session and complications were reported. A kidney-ureter-bladder (KUB) X-ray and ultrasound (US) scan (2 weeks after a single SWL session) were used to assess stone disintegration. Noncontrast computed tomography (NCCT) was done for patients with no residual detected by KUB and US. Stone disintegration was classified as none (no change from basal by KUB X-ray or US imaging), partial (fragmented and >4-mm residual fragments), and complete (≤4-mm residual fragments).
The power of the study was calculated by using the G
*Power program (University of Düsseldorf, Düsseldorf, Germany) to determine an adequate sample size based on previous trials [
1112]. Using the priori test with accuracy mode calculation and an effect size convention of 0.35 for the one-way ANOVA, with α error probability of 0.05, provided 95% power with expected 20% dropout rate for a total sample size of 165 patients. Statistical analysis was performed with the use of the IBM SPSS Statistics ver. 22.0 for Windows (IBM Corp., Armonk, NY, USA). Statistical analyses were done by using one-way ANOVA, and chi-square tests, and use of Bonferroni
post-hoc tests to determine the difference between the two groups. A p-value <0.05 was considered to be statistically significant.
All procedures in this study involving human participants were in accordance with the ethical standards of the Mansoura Faculty of Medicine Institutional Research Board (approval number: MD/161172) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Go to :

RESULTS
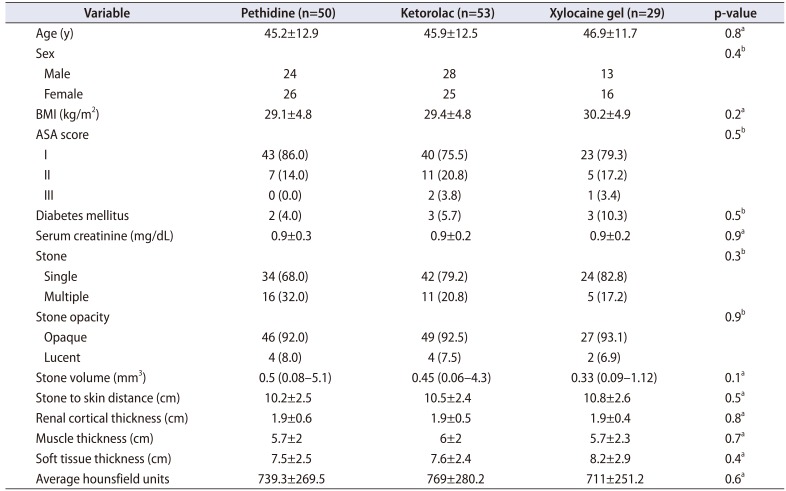
Patient data and stone characteristics of the three groups as shown in
Table 1 did not differ significantly. In the xylocaine group, six sessions (20.7%) were aborted and a supplemental analgesic (our standard regimen was pethidine) was added. Also, most patients in the xylocaine group showed the least satisfaction with pain control; therefore, we stopped allocating patients to this arm. All monitored vital parameters of the patients were comparable between groups.
Table 1
Patient demographic characteristics and stone characteristics in the three groups
|
Variable |
Pethidine (n=50) |
Ketorolac (n=53) |
Xylocaine gel (n=29) |
p-value |
|
Age (y) |
45.2±12.9 |
45.9±12.5 |
46.9±11.7 |
0.8a
|
|
Sex |
|
|
|
0.4b
|
|
Male |
24 |
28 |
13 |
|
|
Female |
26 |
25 |
16 |
|
|
BMI (kg/m2) |
29.1±4.8 |
29.4±4.8 |
30.2±4.9 |
0.2a
|
|
ASA score |
|
|
|
0.5b
|
|
I |
43 (86.0) |
40 (75.5) |
23 (79.3) |
|
|
II |
7 (14.0) |
11 (20.8) |
5 (17.2) |
|
|
III |
0 (0.0) |
2 (3.8) |
1 (3.4) |
|
|
Diabetes mellitus |
2 (4.0) |
3 (5.7) |
3 (10.3) |
0.5b
|
|
Serum creatinine (mg/dL) |
0.9±0.3 |
0.9±0.2 |
0.9±0.2 |
0.9a
|
|
Stone |
|
|
|
0.3b
|
|
Single |
34 (68.0) |
42 (79.2) |
24 (82.8) |
|
|
Multiple |
16 (32.0) |
11 (20.8) |
5 (17.2) |
|
|
Stone opacity |
|
|
|
0.9b
|
|
Opaque |
46 (92.0) |
49 (92.5) |
27 (93.1) |
|
|
Lucent |
4 (8.0) |
4 (7.5) |
2 (6.9) |
|
|
Stone volume (mm3) |
0.5 (0.08–5.1) |
0.45 (0.06–4.3) |
0.33 (0.09–1.12) |
0.1a
|
|
Stone to skin distance (cm) |
10.2±2.5 |
10.5±2.4 |
10.8±2.6 |
0.5a
|
|
Renal cortical thickness (cm) |
1.9±0.6 |
1.9±0.5 |
1.9±0.4 |
0.8a
|
|
Muscle thickness (cm) |
5.7±2 |
6±2 |
5.7±2.3 |
0.7a
|
|
Soft tissue thickness (cm) |
7.5±2.5 |
7.6±2.4 |
8.2±2.9 |
0.4a
|
|
Average hounsfield units |
739.3±269.5 |
769±280.2 |
711±251.2 |
0.6a
|

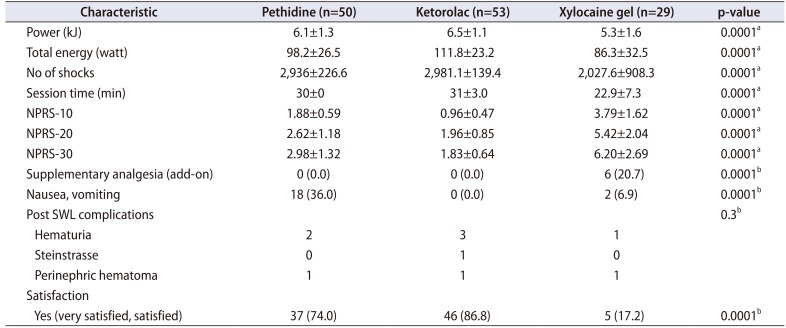
Parameters of the SWL sessions with pain ratings are shown in
Table 2. The NPRS scores were significantly different between groups. By use of the
post-hoc test, NPRS scores were highest in the xylocaine group at 10, 20, and 30 minutes (p=0.0001). However, there was no significant difference between the ketorolac and pethidine groups, except at 10 minutes (p=0.03) and a near significant difference at 30 minutes (p=0.054) in favor of ketorolac. In view of the SWL parameters, the number of shocks and total energy showed some significant differences. By use of the
post-hoc test, patients in the xylocaine group had the least ability to tolerate shock numbers and total energy (p=0.0001), with no significant difference between the ketorolac and pethidine groups (p=1.0) in shocks numbers; however, patients in the ketorolac group tolerated more energy than did patients in the pethidine group (p=0.04).
Table 2
Characteristics of the shock wave sessions and patients' reactions
|
Characteristic |
Pethidine (n=50) |
Ketorolac (n=53) |
Xylocaine gel (n=29) |
p-value |
|
Power (kJ) |
6.1±1.3 |
6.5±1.1 |
5.3±1.6 |
0.0001a
|
|
Total energy (watt) |
98.2±26.5 |
111.8±23.2 |
86.3±32.5 |
0.0001a
|
|
No of shocks |
2,936±226.6 |
2,981.1±139.4 |
2,027.6±908.3 |
0.0001a
|
|
Session time (min) |
30±0 |
31±3.0 |
22.9±7.3 |
0.0001a
|
|
NPRS-10 |
1.88±0.59 |
0.96±0.47 |
3.79±1.62 |
0.0001a
|
|
NPRS-20 |
2.62±1.18 |
1.96±0.85 |
5.42±2.04 |
0.0001a
|
|
NPRS-30 |
2.98±1.32 |
1.83±0.64 |
6.20±2.69 |
0.0001a
|
|
Supplementary analgesia (add-on) |
0 (0.0) |
0 (0.0) |
6 (20.7) |
0.0001b
|
|
Nausea, vomiting |
18 (36.0) |
0 (0.0) |
2 (6.9) |
0.0001b
|
|
Post SWL complications |
|
|
|
0.3b
|
|
Hematuria |
2 |
3 |
1 |
|
|
Steinstrasse |
0 |
1 |
0 |
|
|
Perinephric hematoma |
1 |
1 |
1 |
|
|
Satisfaction |
|
|
|
|
|
Yes (very satisfied, satisfied) |
37 (74.0) |
46 (86.8) |
5 (17.2) |
0.0001b
|

In our RCT, we considered the patients satisfied if their answers were “very satisfied” or “satisfied.” Patient self-reports of satisfaction did not differ significantly between the ketorolac and pethidine groups (p=0.13). Xylocaine had the lowest satisfaction values among the groups (p=0.0001,
Table 2). A total of 36.0% of patients in the pethidine group experienced nausea and/or vomiting compared with only 6.9% of patients in the xylocaine group and none in the ketorolac group (p<0.0001).
Post-SWL complications did not reveal any significant differences in renal hematoma, a drop in hemoglobin, or consequently blood transfusion between the groups (p=0.3). We did not assess the stone-free rate in this RCT, because NCCT was used only in patients who showed complete disintegration (13, 9.8%), whereas US and KUB were done for patients with none and partial disintegration. Also, our policy is to assess the stone-free rate after the last session (maximum n=4) with an interval of 2 weeks in between.
Regarding stone disintegration, the rate was lowest in the xylocaine group, with 26 patients with none (89.7%), 3 with partial (10.3%), and 0 with complete (0.0%) disintegration of the stone (p=0.008). The rate of stone disintegration was better in the ketorolac group than in the pethidine group (p=0.031).
Go to :

DISCUSSION
SWL has revolutionized the treatment of nephrolithiasis worldwide. SWL is a noninvasive or extremely low-invasive treatment that, in most patients, can be carried out without general or regional anesthesia on an outpatient basis [
13]. The success of SWL depends on the efficacy of the lithotripter and the following factors: size, location, and composition of the stones; patient's habitus; and performance of SWL [
5]. Pain control is one of these factors and significantly influences retreatment rate and final outcome of SWL. European guidelines state that careful control of pain during treatment is necessary to limit pain-induced movements and excessive respiratory excursions [
5].
To date, the pathogenesis of SWL pain is not clear. Stimulation of the superficial nociceptors in the skin as well as the deeper, visceral nociceptors in the renal capsule, periosteum, pleura, peritoneum, and muscles caused by the shock wave-generated microbubbles in body fluids play a role in the SWL pain pathway. Moreover, stone movement during the session can increase pain [
14].
Different analgesia regimens have been reported in SWL analgesia practice. Therefore, it is crucial to choose an adequate analgesic with few adverse effects. Sorensen et al. [
15] reported that the 3-month stone-free rate for general anesthesia was 87% compared with a stone-free rate of 55% if IV sedation was used for SWL analgesia (p<0.001). On the contrary, about one third of urologic patients had cardiopulmonary problems, including medically compromised patients or elderly patients that limit the use of standard anesthetic techniques. Sedoanalgesia was developed in urologic surgery in response to the increasing demand for cost-effective, minimally invasive surgery, office surgery, and rapid complication-free recovery [
16].
Pethidine is a synthetic opioid that exerts its analgesic effects by acting as an agonist at µ-receptors. In a doubleblinded RCT, morphine and pethidine provided effective postoperative analgesia with an acceptable adverse effect profile, whereas tramadol had acceptable analgesia but required more rescue analgesic doses [
17]. Although pethidine showed at least a 50% decrease in acute postoperative pain, 68% of patients experienced adverse effects like drowsiness, dizziness, nausea, or vomiting as reported in a systematic review with meta-analysis [
8]. In the current study, the pethidine group had a mean NPRS score of 2.5 regarding pain control. About 36% of patients in the pethidine group experienced adverse effects. The stone disintegration results with pethidine usage were as follows: none, 25 (50.0%); partial, 23 (46.0%); and complete, 2 (4.0%). About three quarters of the patients were satisfied.
Opioid-sparing techniques have been recognized as an important component of postoperative pain management. Ketorolac is a NSAID with analgesic properties. A 60-mg intramuscular injection of ketorolac has a longer time to peak concentration (30–50 minutes) than does a 30-mg IV dose (3–5 minutes). The 60-mg dose reduces early postoperative pain, has opioid-sparing effects, and reduces postoperative nausea and vomiting, with lack of evidence of these effects for the 30-mg dose. Regarding safety, ketorolac does not show an increase in abnormal bleeding, blood transfusions, or gastritis symptoms [
9]. In our RCT, ketorolac did not show a significant increase in renal hematoma, a drop in hemoglobin, or consequent blood transfusion.
In a systematic review with meta-analysis by Smith et al. [
8], for single-dose ketorolac and pethidine in acute postoperative pain, the authors found that the number-needed-to-treat (NNT) for pethidine 100 mg was 2.9 (95% confidence interval [CI], 3.2–3.9), and 53.6% had at least 50% pain relief. The NNT for 30-mg ketorolac was 3.4 (95% CI, 2.5–4.9), and 52.8% of patients had at least 50% pain relief. However, with the 60-mg ketorolac dose, the NNT was 1.8 (95% CI, 1.5–2.3), and 55.5% of patients had at least 50% pain relief.
In our series, the ketorolac group had a tolerable pain rating with no reported adverse effects during SWL sessions. Ketorolac had favorable stone disintegration results: none, 19 (35.8%); partial, 23 (43.4%); and complete, 11 (20.8%), respectively (p=0.008). Patient satisfaction was more than 85%. Akcali et al. [
18] concluded that lornoxicam, an NSAID, can be safely and efficiently preferred in pain control during SWL. In a recent meta-analysis that assessed analgesia for patients undergoing SWL, no significant difference in pain scores was reported between NSAIDs or opioids [
4].
EMLA cream containing lidocaine and prilocaine is a topical anesthetic drug designed for use on intact skin. EMLA cream has been used for SWL sedoanalgesia with conflicting results [
1219]. In one RCT, EMLA cream was reported to be as safe and effective as fentanyl, diclofenac, and tramadol with no statistically significant differences regarding pain scores or adverse effects [
12]. However, in the other RCT, EMLA cream did not significantly modify pain perceived and did not have any significant opioid-sparing effect [
19]. In our study, we used lidocaine instead of EMLA cream owing to its availability in our center.
Lidocaine requires an injection, a painful process, to conduct deep anesthesia. Transdermal drug delivery, known as sonophoresis, is a noninvasive alternative to local injection by use of US [
20]. Luh et al. [
21] proved that pretreatment extracorporeal shock wave therapy (ESWT) and concurrent ESWT accelerate the anesthetic effects of local anesthetic. In this study, xylocaine (lidocaine) gel had the highest NPRS scores. A total of 20% of patients stopped the SWL session due to pain. Patient satisfaction was only 17% in the xylocaine group. This higher rate of pain associated with patient movement, beside the aborted SWL in 6 sessions (20.7%), might have caused the unfavorable stone disintegration results in the xylocaine group, which were as follows: none, 26 (89.7%); partial, 3 (10.3%); and complete, 0 (0.0%), respectively (p=0.008).
Although our study was an RCT, it had some limitations. Not all patients underwent NCCT for assessment of the stone-free rate. Also, we tested these drugs during a single SWL session in a small number of patients. Patient-reported satisfaction was not assessed by use of a validated questionnaire. Additional larger trials are needed to assess a combination of multiple regimens of analgesia in a large number of patients.
Go to :




