Abstract
Background
The natural course of thyroid cancer nodules and benign nodules is different. This study was to compare the changes in size between thyroid cancer nodules and thyroid benign nodules. The risk factors associated with the changes of thyroid cancer nodules were assessed.
Methods
This study contains retrospective observational and prospective analysis. A total of 113 patients with 120 nodules were recruited in the cancer group, and 116 patients with 119 nodules were enrolled in the benign group. Thyroid ultrasonography was performed at least two times at more than 1-year interval.
Results
The mean follow-up durations were 29.5±18.8 months (cancer group) and 31.9±15.8 months (benign group) (P=0.32). The maximum diameter change in length was 0.36±0.97 mm/year in the cancer group and –0.04±0.77 mm/year in the benign group (P<0.01). The volume was significantly increased in the cancer group compared with the benign group (0.06±0.18 mL/year vs. 0.004±0.05 mL/year, respectively, P<0.01; 26.9%±57.9%/year vs. 1.7%±26.0%/year, P<0.01). Initial maximum diameter (β=0.02, P<0.01) and initial volume (β=0.13, P<0.01) were significantly associated with volume change (mL)/year. Initial maximum standardized uptake value did not predict the nodule growth.
Thyroid cancer is the most common cancer in South Korean women [1]. It is an indolent disease with a low mortality rate. Additionally, several published autopsy studies have reported that the small occult thyroid cancer can be detected with a high degree of probability [234]. Small thyroid cancer without high-risk factors does not have any symptoms and affect survival rate. However, surgery is considered the optimal treatment for thyroid cancer immediately after diagnosis. Therefore, its natural course remains unclear. Only a small number of thyroid cancers have an aggressive prognosis. So, identifying the risk factors for poor prognosis in thyroid cancer before surgery are very important.
In several studies, papillary thyroid microcarcinomas (i.e., papillary thyroid carcinomas measuring ≤10 mm) diagnosed using ultrasonography (US) guided fine-needle aspiration biopsy (FNAB) were not surgically treated immediately after diagnosis [56]. According to an observational study in Japan, nodules 3 mm in size or larger increased 6.4% after 5 years and 15.9% after 10 years. In another study, 22 of 300 patients (7%) showed nodules 3 mm in size or larger increased during the follow-up period. In studies regarding the predictors of thyroid cancer growth, thyroid-stimulating hormone (TSH) and tumor progression were not significantly associated [7]. In another study, patients under the age of 40 years showed a high incidence of node size enlargement and metastasis [8].
In this study, we evaluated changes in the length and volume of thyroid cancer nodules identified using FNAB or surgery and compared the change in size between thyroid cancer and benign nodules. Additionally, we analyzed the risk factors associated with the growth of thyroid cancer. The initial mean age, gender, TSH levels, autoantibody presence, initial nodule size, taller-than-wide shape, and microcalcification were considered as risk factors for rapid progression of thyroid cancer nodule. To accurately analyze the change in size, we calculated the nodule volumes. In previous studies, to compare the nodule size increase, only a single thyroid US section was used. We analyzed the association of high maximum standardized uptake value (SUV) and thyroid nodule growth in several patients who underwent preoperative [18F] Fluoro-2-D-deoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) scan.
The present study was conducted at Yeouido St. Mary's Hospital and Seoul St. Mary's Hospital in Seoul, Korea. Inclusion criteria for the thyroid cancer group were the following: thyroid nodules confirmed using FNAB or surgery; FNAB results classified according to the Bethesda system; thyroid nodules diagnosed using FNAB defined as cancer nodules if the result either Bethesda V (suspicious for malignancy) or Bethesda VI (malignant); the patients underwent thyroid US at least two times at more than 1-year intervals (range, 12.5 to 47.5 months) before surgery.
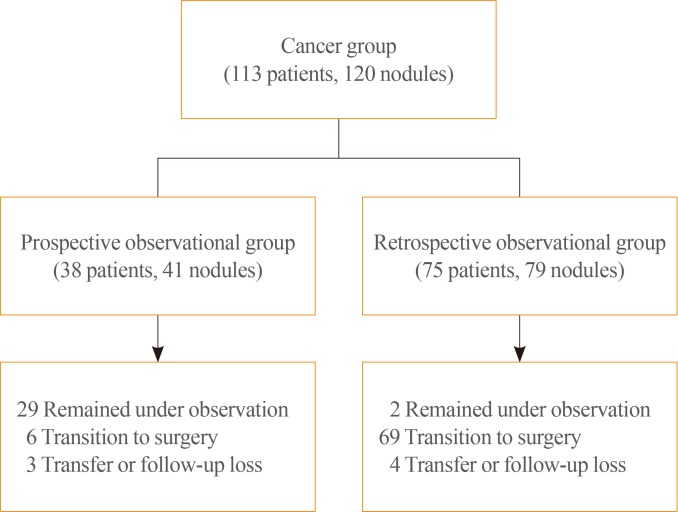
The cancer group was divided into the prospective and retrospective observational groups. Thirty-eight patients (41 nodules) in the prospective observational group received follow-up thyroid US after FNAB-based diagnosis. These were all Bethesda V (suspicious for malignancy) or Bethesda VI (malignant). The retrospective observational group included 75 patients (79 nodules) who were diagnosed based on FNAB or surgery; Sixty-eight nodules were confirmed as Bethesda V or VI based on FNAB. Eleven nodules were non-diagnostic/atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) by FNAB and confirmed as papillary thyroid carcinoma later by surgery. A total of 113 patients and 120 nodules were enrolled in the present study (Fig. 1).
Among the enrolled subjects, 75 underwent thyroidectomy. All thyroid nodules were confirmed as papillary thyroid carcinoma after surgery. If lymph node metastasis was invisible on thyroid US, thyroid nodules were observed without surgical resection. A total of 31 patients underwent nonsurgical observation.
The benign group prospectively included patients with benign thyroid nodules identified using FNAB who underwent thyroid US at least twice at more than 1-year intervals. Cystic or predominantly cystic nodules (cystic portion of thyroid nodule at initial US >50%) were excluded. The size of nodules was matched to compare with the nodules of the cancer group. A total of 116 patients (119 nodules) were included in this group.
Thyroid US was performed using 3000 or 5000 scanners (ATL, Philips, Bothell, WA, USA) with a 5 to 14 MHz transducer. Thyroid US was performed by radiologist or endocrinologist in our institution and was reviewed and confirmed by one of our researchers (K.J.Y.). Depth and width were measured from the transverse view and length from the longitudinal view. The volume of the nodule was calculated using the formula for rotational ellipsoid; depth (cm)×width (cm)×length (cm)×π/6 [9]. The nodule maximum diameter was defined as the longest diameter of the three measurements (depth, width, length). Taller than wide was defined as an anteroposterior/transverse diameter (A/T) ratio >1 in the transverse view, and A/T ratio was also calculated. Calcification was considered as the presence of microcalcification. Cohen' κ value was calculated to confirm the interobserver variability. The serum TSH (Beckman Coulter, Brea, CA, USA; normal range, 0.55 to 4.78 µIU/mL), thyroid peroxidase antibody (TPO Ab; Roche Diagnostics, Rotkreuz, Switzerland; normal range, 0 to 34 U/mL), and thyroglobulin antibody (Tg Ab; ZenTech, Angleur, Belgium; normal range, 0 to 115 U/mL) were measured using chemiluminescent immunoassays.
Before surgery, 43 patients received a 18F-FDG PET/CT. Two experienced nuclear medicine physicians reviewed the 18F-FDG PET/CT. The maximum SUV was calculated in a region of interest. This study was approved by the Institutional Review Board of Yeouido St. Mary's Hospital (SC15RISI0047), and all patients signed informed consents before entering the study.
All data processing was performed with SPSS software version 18.0 software (SPSS Inc., Chicago, IL, USA). t test and chi-square test were used to compare the baseline clinical characteristics and thyroid nodule change in the cancer and benign groups. The data were presented as mean±standard deviation for continuous variables and as proportions for categorical variables. Multiple linear regression analysis was performed to confirm progression risk factors for thyroid cancer nodules. The initial mean age, gender, TSH levels, autoantibody presence, initial maximum diameter, initial volume, depth/width ratio, and microcalcification were included as risk factors. The initial maximum diameter and initial volume were analyzed separately because of multicollinearity. To identify the relationships between pathologic findings and rapid progression of thyroid cancer nodules, the chi-square test was used. A P<0.05 was considered to indicate statistical significance.
The clinical baseline characteristics of the study population are presented in Table 1. The initial mean age, gender, TSH levels, TPO Ab levels, Tg Ab levels, number of FNABs, initial maximum diameter, and initial volume did not differ between the two groups. The taller than wide, A/T ratio, and microcalcification finding on thyroid US appeared more frequently in the cancer group. FNAB results in the cancer group were nondiagnostic or unsatisfactory or AUS/FLUS (11.8%), suspicious for malignancy (33.3%), and malignancy (51.0%).
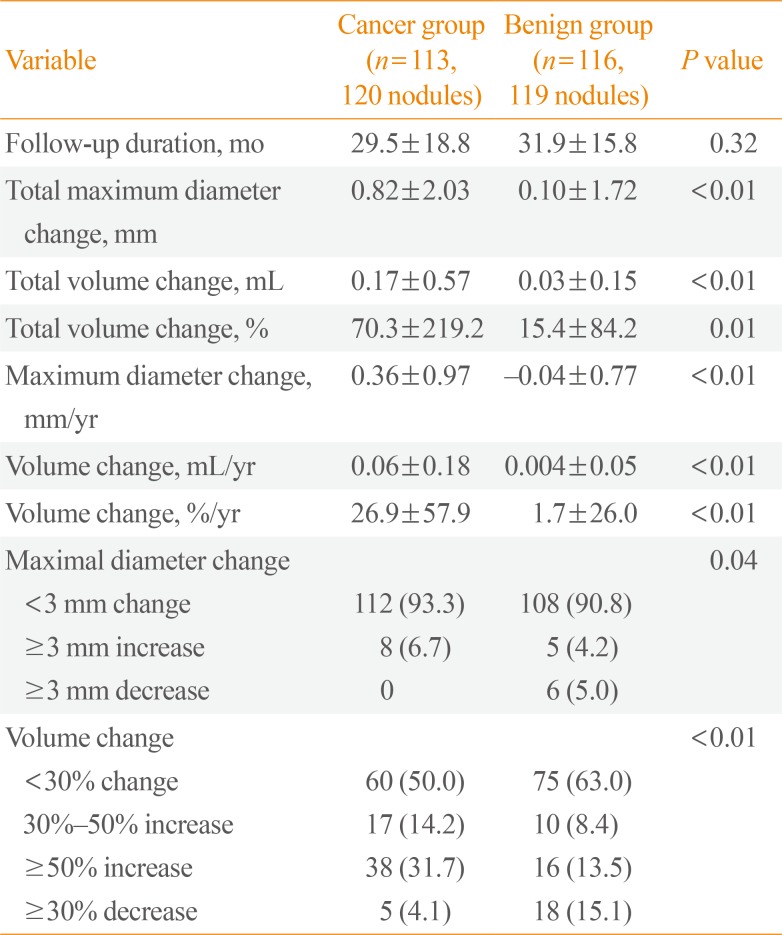
The mean follow-up durations in the cancer group and the benign group were 29.5±18.8 months (range, 12 to 94) and 31.9±15.8 months (range, 12 to 85), respectively (P=0.32) (Table 2). We calculated maximum diameter change (mm)/year, volume change (mL)/year and volume change (%)/year for adjusting duration. The maximum diameter change (mm)/year was 0.36±0.97 mm in the cancer group and –0.04±0.77 mm in the benign group (P<0.01). Additionally, the volume change (mL)/year and volume change (%)/year were significantly increased in the cancer group compared with the benign group. The volume change (%)/year were 26.9%±57.9% in the cancer group and 1.7%±26.0% in the benign group (P<0.01). The initial nodule volume increased 50% or more during follow-up in 31.7% of the cancer group patients and 13.5% of the benign group patients. The patients in the prospective observational group underwent thyroid US every 6 or 12 months. New lymph node metastasis in the left level VI was found only in one patient whose FNAB results of suspicious lymph node included a few clusters of atypical cells, suspicious of metastatic carcinoma. The patient underwent total thyroidectomy with dissection of both central lymph nodes.
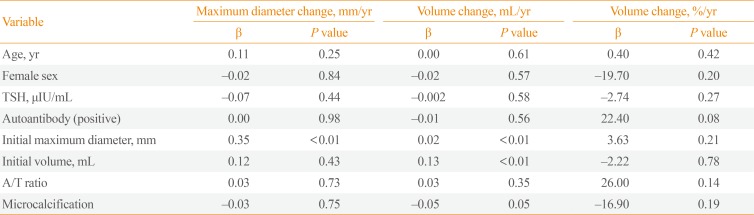
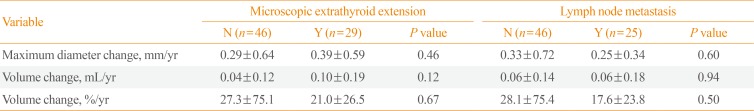
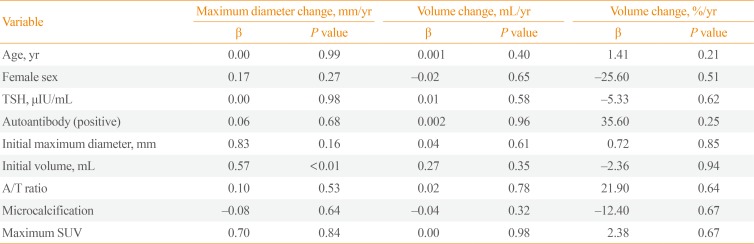
Multiple linear regression analysis was performed to determine the risk factors of rapid progression of thyroid cancer nodules (Table 3). The initial age, gender, TSH levels, thyroid autoantibody levels, and microcalcification were not associated with volume change (mL)/year and volume change (%)/year. However, initial maximum diameter (β=0.02, P<0.01) and initial volume (β=0.13, P<0.01) were significantly associated with volume change (mL)/year. The pathologic findings were confirmed in 75 patients after surgery. To verify the relevance of the thyroid cancer nodule growth and pathologic findings, the patients were divided into two groups based on the presence of microscopic extrathyroid extension (ETE) or lymph node metastasis (Table 4). However, microscopic ETE and lymph node metastasis were not associated with tumor volume growth.
We performed subgroup analyses of the 43 patients who received preoperative 18F-FDG PET/CT. The total maximum SUV average was 3.51±2.91. The maximum SUVs were not associated with volume change (mL)/year and volume change (%)/year after adjusting for initial mean age, gender, TSH levels, thyroid autoantibody presence, initial maximum diameter, initial volume, depth/width ratio, and microcalcification in the multiple linear regression analysis (Table 5). Additionally, the maximum SUV depending on the presence or absence of lymph node metastasis and microscopic ETE was not significantly different (data not shown).
According to the American Thyroid Association guideline [10], significant thyroid nodule size change was defined as more than 50% volume increase or 2 mm and 20% nodule diameter increase in at least two nodule dimensions. In the present study, we compared the size change of cancer nodules and benign nodules during an average follow-up of 30.1±17.1 months. The maximum diameter change/year was 0.36±0.97 mm in the cancer group and –0.04±0.77 mm in the benign group (P<0.01). In addition, volume change (%)/year was significantly increased in the cancer group compared with the benign group (26.9%±57.9% vs. 1.7%±26.0%, P<0.01). Initial maximum diameter and initial volume were independent risk factors for cancer nodule growth in the multiple regression analysis. However, there was no association between cancer nodule growth and pathologic findings.
In the present study, the annual maximum diameter change in the cancer nodules was approximately only 0.36 mm. However, the annual volume change showed a significant increase of approximately 27%. The nodule diameter observed on thyroid US single plane may vary depending on the examiner's skill or angle in the thyroid US plane. Therefore, evaluating the size change by comparing the length difference of the nodule in a single US section is difficult. Thyroid nodule growth was defined as 3-mm change in diameter in several observational studies [5611]. However, measuring the volume of thyroid nodules to assess the size change would be more accurate. This finding is consistent with the previous studies [121314].
Several studies regarding the volume change of benign thyroid nodules using thyroid US have been performed [15161718]. When using 50% as a cut-off value, 10% to 15% of thyroid nodules increased in volume after 20 months of follow-up. When using 30% as a cut-off value, 25% to 50% of thyroid nodules increased in volume after 40 to 60 months of follow-up. In the present study, when using 50% and 30% as cut-off values in the benign group, 13.5% and 15.1% of thyroid nodules increased in volume, respectively. These results were not significantly different from other studies. When using 50% and 30% as cut-off values in the cancer group, 31.7% and 4.1% of thyroid nodules increased in volume, respectively. These results show a two-fold difference between the two groups.
The risk factors for thyroid cancer nodule growth are still controversial. Recent studies showed that higher serum TSH is associated with a higher incidence of thyroid cancer, advanced stage thyroid cancer, and tumor growth during active surveillance [1920212223]. Other studies showed thyroid autoimmunity such as positive Tg Ab is a risk factor for thyroid cancer [2024]. However, in prospective cohort studies in Japan, size progression of thyroid cancer was not associated with TSH levels and thyroid autoimmunity [67]. Ito et al. [8] reported that younger-aged (<40 years) patients exhibited more rapid progression of thyroid cancer and node metastasis. Recently, Oh et al. [12] also reported active surveillance should be carefully applied for younger patients. According to our results, age, gender, TSH levels, and thyroid autoantibody were not associated with thyroid cancer nodule growth. Initial size was an independent risk factor for cancer nodule growth. Several studies reported that initial tumor size had no effect on thyroid cancer progression [67]. However, in the present study the initial tumor volume was included and adjusted for several related risk factors. This is consistent with the recently published study by Oh et al. [12]. Further high-quality prospective studies regarding initial tumor size and progression are needed.
18F-FDG PET/CT is an imaging tool using the characteristics of increased glucose metabolism in cancer cells [25]. FDG uptake in 18F-FDG PET/CT is a useful predictor for prognosis and metastasis in many malignant tumors [262728]. Several published studies showed 18F-FDG PET/CT were effective for the prediction of recurrence and prognosis after thyroidectomy [2930]. However, whether FDG positivity in primary thyroid cancer is effective for predicting prognosis remains unknown. In several studies, the FDG positive group showed a higher prevalence of microscopic ETE and central lymph node involvement compared with the FDG negative group [3132]. In contrast, other studies reported that preoperative FDG uptake in thyroid cancer was not associated with postoperative pathology [3334]. We performed subgroup analyses in patients receiving preoperative 18F-FDG PET/CT. Maximum SUV values did not affect the nodule growth and were not associated with lymph node metastasis and microscopic ETE.
The present study had several strengths. First, we compared the nodule growth in both the cancer and benign groups. Additionally, the baseline characteristics between the two groups did not significantly differ. Second, several risk factors including maximum SUV values for thyroid cancer nodule growth were analyzed. However, the present study had several limitations. First, the sample size was small. Second, thyroid nodules belonging to the inclusion criteria were selected; therefore, a selection bias may have existed. Third, benign nodules and some cancer nodules were diagnosed with non-surgical, FNAB. However, FNAB is a highly accurate diagnostic method. Several studies reported that the sensitivity and specificity levels of FNAB were 80% to 90% and 94% to 98%, respectively [353637]. Fourth, the number of FNABs performed could have affected the change in nodule size. However, the numbers of FNABs performed in the two groups were similar. Additionally, predominantly cystic or cystic nodules were excluded in the benign group because the nodule size may be susceptible to FNABs. Finally, this study included relatively small thyroid nodules, which caused concern about the biased measurement. In conclusion, the thyroid cancer nodules showed rapid progression compared with benign nodules. The initial maximum diameter and initial volume were independent risk factors for cancer nodule growth.
References
1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14. PMID: 23613665.

2. Sampson RJ, Woolner LB, Bahn RC, Kurland LT. Occult thyroid carcinoma in Olmsted County, Minnesota: prevalence at autopsy compared with that in Hiroshima and Nagasaki, Japan. Cancer. 1974; 34:2072–2076. PMID: 4474057.

3. Sampson RJ, Key CR, Buncher CR, Iijima S. Thyroid carcinoma in Hiroshima and Nagasaki. I. Prevalence of thyroid carcinoma at autopsy. JAMA. 1969; 209:65–70. PMID: 5819259.

4. Bondeson L, Ljungberg O. Occult thyroid carcinoma at autopsy in Malmo, Sweden. Cancer. 1981; 47:319–323. PMID: 7459819.
5. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010; 34:1222–1231. PMID: 20066418.

6. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010; 34:28–35. PMID: 20020290.

7. Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014; 38:673–678. PMID: 24233662.

8. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24:27–34. PMID: 24001104.

9. Knudsen N, Bols B, Bulow I, Jorgensen T, Perrild H, Ovesen L, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999; 9:1069–1074. PMID: 10595454.

10. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214. PMID: 19860577.

11. Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007; 3:240–248. PMID: 17315032.

12. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid. 2018; 28:1587–1594. PMID: 30226447.

13. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center's experience in Korea. J Clin Endocrinol Metab. 2017; 102:1917–1925. PMID: 28323932.

14. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017; 143:1015–1020. PMID: 28859191.

15. Quadbeck B, Pruellage J, Roggenbuck U, Hirche H, Janssen OE, Mann K, et al. Long-term follow-up of thyroid nodule growth. Exp Clin Endocrinol Diabetes. 2002; 110:348–354. PMID: 12397534.

16. Lim DJ, Kim JY, Baek KH, Kim MK, Park WC, Lee JM, et al. Natural course of cytologically benign thyroid nodules: observation of ultrasonographic changes. Endocrinol Metab (Seoul). 2013; 28:110–118. PMID: 24396664.

17. Erdogan MF, Gursoy A, Erdogan G. Natural course of benign thyroid nodules in a moderately iodine-deficient area. Clin Endocrinol (Oxf). 2006; 65:767–771. PMID: 17121528.

18. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003; 138:315–318. PMID: 12585829.

19. McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2012; 97:2682–2692. PMID: 22622023.

20. Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK, Kwon HS, et al. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid. 2010; 20:885–891. PMID: 20465529.

21. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008; 93:809–814. PMID: 18160464.

22. Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf). 2009; 71:434–439. PMID: 19067720.

23. Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High serum TSH level is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab. 2018; 103:446–451. PMID: 29211863.

24. Vasileiadis I, Boutzios G, Charitoudis G, Koukoulioti E, Karatzas T. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann Surg Oncol. 2014; 21:2725–2732. PMID: 24595799.

25. Buerkle A, Weber WA. Imaging of tumor glucose utilization with positron emission tomography. Cancer Metastasis Rev. 2008; 27:545–554. PMID: 18523732.

26. Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004; 5:531–540. PMID: 15337482.

27. Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007; 110:1738–1744. PMID: 17786947.

28. Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005; 35:84–99. PMID: 15765372.

29. Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000; 85:1107–1113. PMID: 10720047.

30. Feine U, Lietzenmayer R, Hanke JP, Held J, Wohrle H, Muller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996; 37:1468–1472. PMID: 8790195.
31. Yun M, Noh TW, Cho A, Choi YJ, Hong SW, Park CS, et al. Visually discernible [18F]fluorodeoxyglucose uptake in papillary thyroid microcarcinoma: a potential new risk factor. J Clin Endocrinol Metab. 2010; 95:3182–3188. PMID: 20427505.

32. Mitchell JC, Grant F, Evenson AR, Parker JA, Hasselgren PO, Parangi S. Preoperative evaluation of thyroid nodules with 18FDG-PET/CT. Surgery. 2005; 138:1166–1174. PMID: 16360405.

33. Kim MH, Ko SH, Bae JS, Lee SH, Jung CK, Lim DJ, et al. Non-FDG-avid primary papillary thyroid carcinoma may not differ from FDG-avid papillary thyroid carcinoma. Thyroid. 2013; 23:1452–1460. PMID: 23688271.

34. Jeong HS, Chung M, Baek CH, Ko YH, Choi JY, Son YI. Can [18F]-fluorodeoxyglucose standardized uptake values of PET imaging predict pathologic extrathyroid invasion of thyroid papillary microcarcinomas? Laryngoscope. 2006; 116:2133–2137. PMID: 17146385.

35. Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst. 2012; 24:63–70. PMID: 23582597.

36. Ko HM, Jhu IK, Yang SH, Lee JH, Nam JH, Juhng SW, et al. Clinicopathologic analysis of fine needle aspiration cytology of the thyroid. A review of 1,613 cases and correlation with histopathologic diagnoses. Acta Cytol. 2003; 47:727–732. PMID: 14526669.
37. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine-needle aspiration of thyroid. Arch Pathol Lab Med. 2001; 125:484–488. PMID: 11260620.

Table 1
Baseline Clinical Characteristics of Total Population
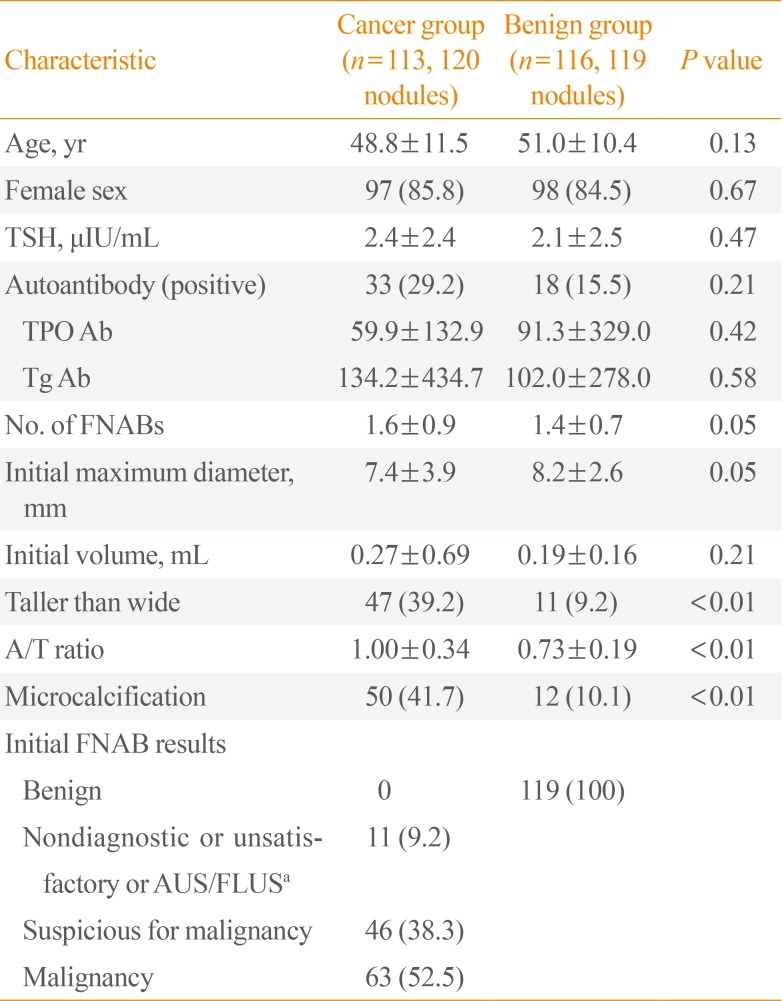
Values are expressed as mean±SD or number (%).
TSH, thyroid stimulating hormone; TPO Ab, thyroid peroxidase antibody; Tg Ab, thyroglobulin antibody; FNAB, fine-needle aspiration biopsy; A/T ratio, anteroposterior/transverse diameter ratio in the transverse view; AUS/FLUS, Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance.
aIt is the result of FNAB before surgery. AUS/FLUS in cancer group were histologically confirmed as classic papillary thyroid carcinoma by surgery.
Table 3
Risk Factors for Progression of Thyroid Cancer Nodules in Multiple Linear Regression Analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download