Abstract
Background
Flow cytometry analysis of paroxysmal nocturnal hemoglobinuria (PNH) is significantly affected by the methodology used. The lack of data on the effect of age and refrigeration on PNH clone stability motivated us to study these aspects using flow cytometry.
Methods
Peripheral blood was collected from six patients, of which two presented with PNH. All samples were tested immediately and stored at room temperature (RT, 20–25˚C) and at 4˚C for re-analysis at 24, 48, 72 hr and 7 days. Anti-CD59-fluorescein isothiocyanate (Beckman Coulter, USA) and anti-CD235a-phycoerythrin (PE; Beckman Coulter) were used to stain red blood cells (RBCs). Fluorescein-labeled proaerolysin (Cedar-lane, Canada), anti-CD15-PE (Beckman Coulter), anti-CD24-PE-cyanin 5 (Beckman Coulter), and anti-CD45-PE-cyanin 7 (Beckman Coulter) were used to stain granulocytes. Flow cytometry was performed using a FC500 flow cytometer (Beckman Coulter). The effects of time and temperature were analyzed using generalized estimating equations.
Results
No significant differences in the gated percentage of RBCs and PNH clone size of RBCs were observed between the RT and 4˚C groups up to 7 days of testing. The percentage of gated neutrophils decreased with specimen age (P<0.001) and a better correlation with baseline was obtained at 4˚C than at RT (P=0.014). Neutrophil PNH clones were stable until 48 hr and 72 hr at RT and 4˚C, respectively, and could not be analyzed at 7 days.
References
1. Nakakuma H. Mechanism of intravascular hemolysis in paroxysmal nocturnal hemoglobinuria (PNH). Am J Hematol. 1996; 53:22–9.

2. Borowitz MJ, Craig FE, Digiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, et al. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by fow cytometry. Cytometry B Clin Cytom. 2010; 78:211–30.
3. Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004; 350:552–9.

4. Hillmen P, Muus P, Duhrsen U, Risitano AM, Schubert J, Luzzatto L, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007; 110:4123–8.

5. Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008; 111:1840–7.

6. Sreedharanunni S, Sachdeva MU, Bose P, Varma N, Bansal D, Trehan A. Frequency of paroxysmal nocturnal hemoglobinuria clones by multiparametric fow cytometry in pediatric aplastic anemia patients of Indian ethnic origin. Pediatr Blood Cancer. 2016; 63:93–7.
7. Sipol AA, Babenko EV, Borisov VI, Naumova EV, Boyakova EV, Yakunin DI, et al. An interlaboratory comparison of PNH clone detection by high-sensitivity fow cytometry in a Russian cohort. Hematology. 2015; 20:31–8.
8. Sachdeva MU, Varma N, Chandra D, Bose P, Malhotra P, Varma S. Multiparameter FLAER-based fow cytometry for screening of paroxysmal nocturnal hemoglobinuria enhances detection rates in patients with aplastic anemia. Ann Hematol. 2015; 94:721–8.
9. Sutherland DR, Keeney M, Illingworth A. Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by fow cytometry. Cytometry B Clin Cytom. 2012; 82:195–208.
10. Donohue RE, Marcogliese AN, Sasa GS, Elghetany MT, Redkar AA, Bertuch AA, et al. Standardized high-sensitivity fow cytometry testing for paroxysmal nocturnal hemoglobinuria in children with acquired bone marrow failure disorders: A single center US study. Cytometry B Clin Cytom. 2018; 94:699–704.
Fig. 1.
Comparison of (A) gated RBCs (%), (B) paroxysmal nocturnal hemoglobinuria (PNH) clones of RBCs (%), (C) gated neutrophils (%), and (D) PNH clones of neutrophils (%) between the room temperature group and the 4°C group according to time in flow cytometry analysis. Generalized estimating equation tests were used.
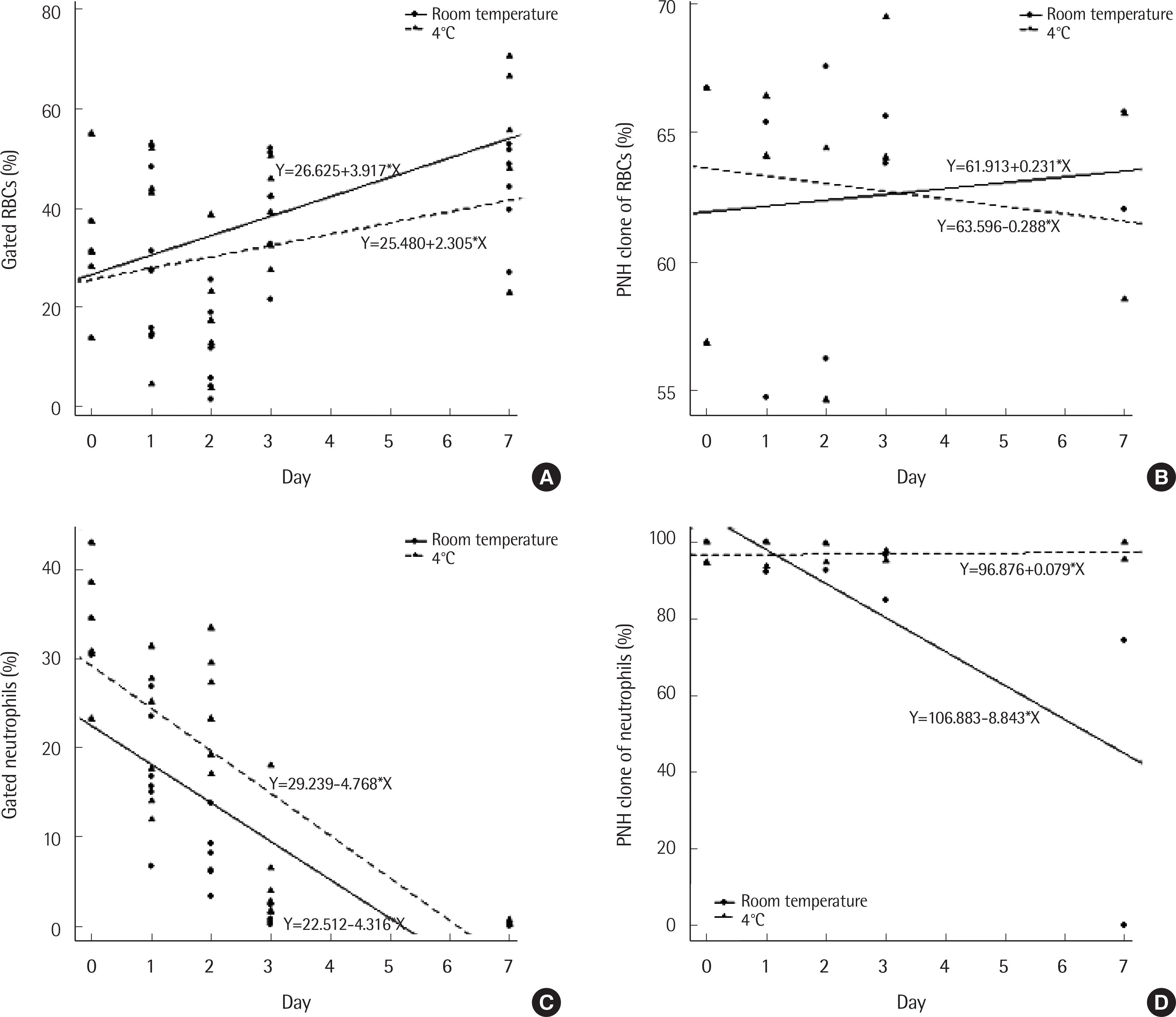
Fig. 2.
Paroxysmal nocturnal hemoglobinuria (PNH) red blood cell analysis according to testing time in a PNH patient (Case 2 in Table 1). A blood sample was tested within 1 hr of collection and stored at room temperature (A) and at 4°C (B). Reanalysis was performed at 24 hr, 48 hr, 72 hr, and 7 days. The size of PNH clone (II+III) of RBCs was 56.85% and showed no effect due to time or temperature. At 7 days, the PNH clone was 65.78% (room temperature) and 58.54% (4°C).
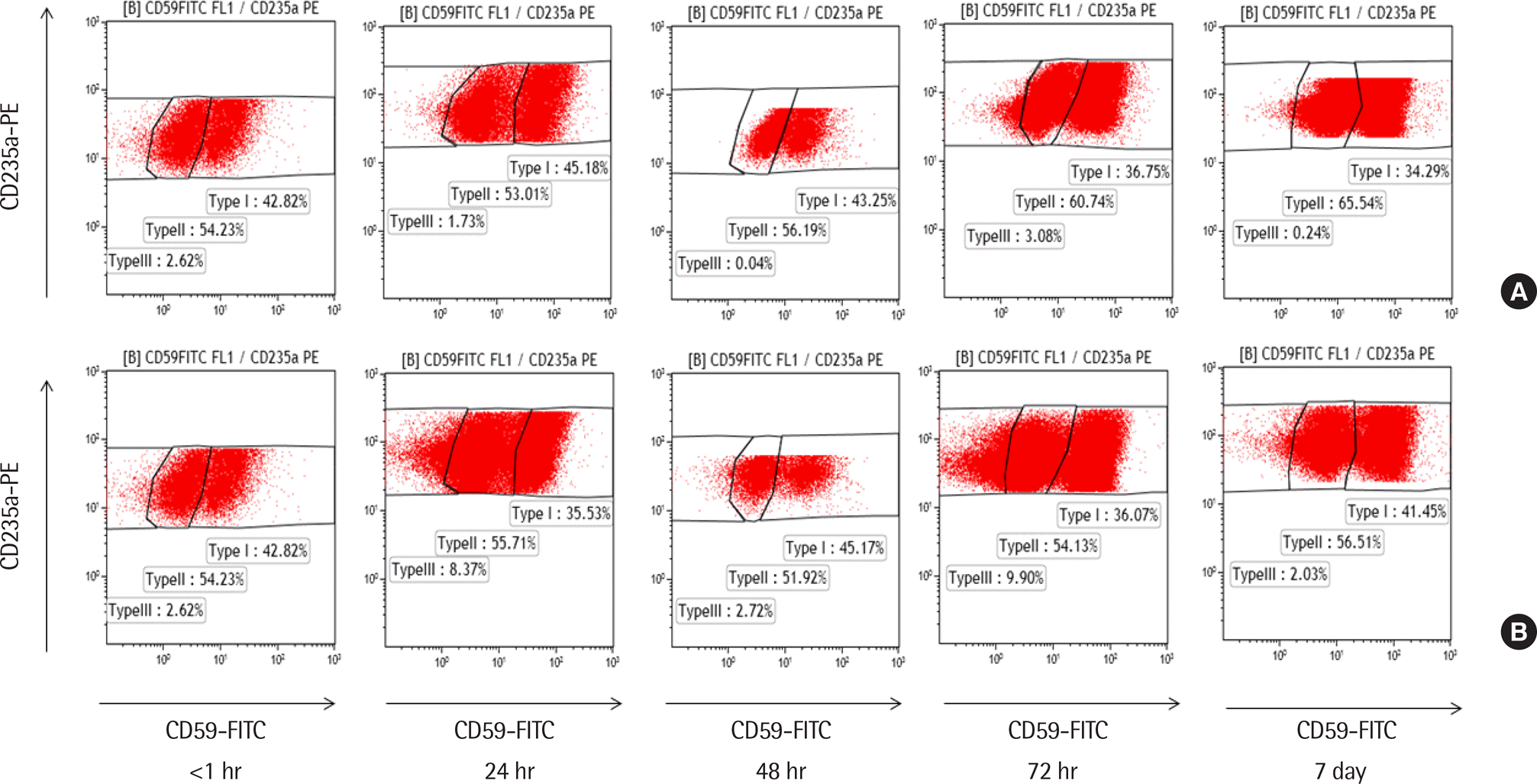
Fig. 3.
Paroxysmal nocturnal hemoglobinuria (PNH) neutrophil analysis according to testing time in a PNH patient (Case 2 in Table 1). A blood sample was tested within 1 hr of collection and stored at room temperature (A) and at 4°C (B). Reanalysis was performed at 24 hr, 48 hr, 72 hr, and 7 days. The size of the PNH clone (II+III) of neutrophils was 94.59%. The clone was stable until 48 hr at room temperature and until 72 hr at 4°C.
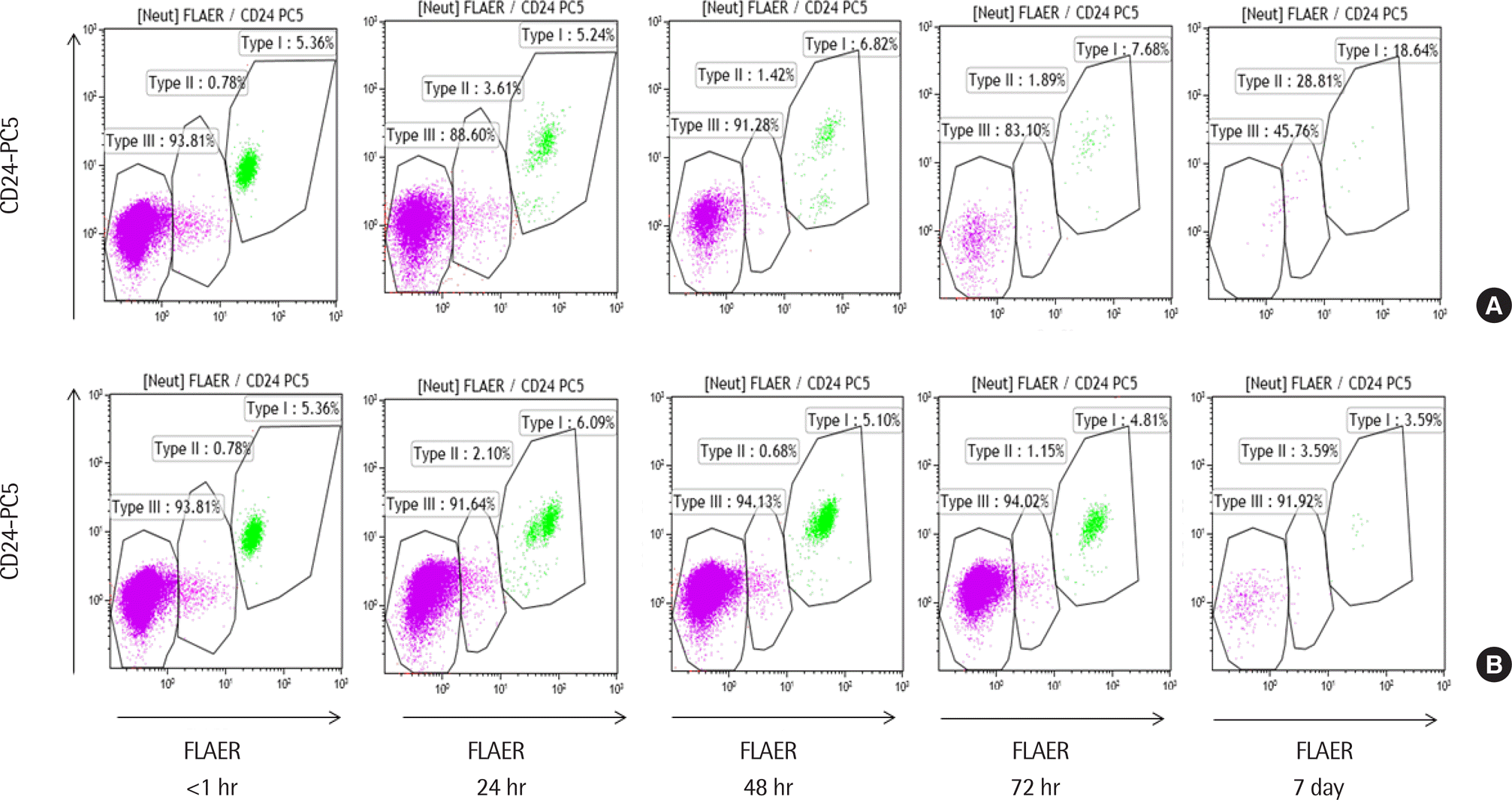
Table 1.
Comparison of paroxysmal nocturnal hemoglobinuria (PNH) cells of two PNH patients according to time and temperature of specimen storage in flow cytometric analyses




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download