Abstract
Iso-oncotic human serum albumin (HSA) is the primary replacement fluid of choice during therapeutic plasma exchange (TPE). Hypersensitivity reactions to HSA are rare, but require proper evaluation and management. In this article, we report two cases of hypersensitivity reactions to 5% HSA during TPE and discuss strategies to address this problem. The first case was a 60-year-old female patient, who was scheduled for TPE for treatment of recurrent focal segmental glomerulosclerosis after ABO-incompatible kidney transplantation. She developed a pruritic rash on her entire body during the first two sessions of TPE using 5% HSA. The third session was conducted using 500 mL normal saline, 1,000 mL 10% pentastarch, and 750 mL 5% HSA, where she eventually developed a pruritic rash when HSA was infused. There were no adverse events during the fourth and fifth session when fresh frozen plasma was used in place of HSA. The second case was a 50-year-old male patient diagnosed with optic neuritis, who was admitted for five sessions of TPE. The patient developed a pruritic rash on his entire body during the first session of TPE using 5% HSA. The patient experienced no adverse events during the following four sessions using fresh frozen plasma. Certain elements contained in HSA, such as albumin aggregates, prekallikrein activator, and caprylate-modified albumin, might be the reason for these hypersensitivity reactions. Careful selection of alternative replacement fluids is important to avoid premature termination of TPE procedures and secure optimal treatment options for patients.
Go to : 
References
1. McLeod BC. Therapeutic apheresis: use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract Res Clin Haematol. 2006; 19:157–67.

2. Pandey S and Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(S1):65S–79S.
3. Fung MK, Grossman BJ, et al. eds. Technical manual. 18th ed.Bethesda: American Association of Blood Banks;2006. p. 678–80.
4. Stafford CT, Lobel SA, Fruge BC, Mofftt JE, Hoff RG, Fadel HE. Anaphylaxis to human serum albumin. Ann Allergy. 1988; 61:85–8.
5. Komericki P, Grims RH, Aberer W, Kränke B. Near-fatal anaphylaxis caused by human serum albumin in fbrinogen and erythrocyte concentrates. Anaesthesia. 2014; 69:176–8.
6. Fujita A, Kitayama M, Hirota K. Anaphylactoid shock in a patient following 5% human serum albumin infusion during off-pump coronary artery bypass grafting. J Anesth. 2007; 21:396–8.

7. Ring J, Stephan W, Brendel W. Anaphylactoid reactions to infusions of plasma protein and human serum albumin. Role of aggregated proteins and of stabilizers added during production. Clin Allergy. 1979; 9:89–97.

8. Beavers C, Flinchum D, Ayyoubi MT. Severe intraoperative albumin transfusion reaction and review of the literature. Lab Med. 2013; 44:e129–31.

9. Jung SY, Choi YJ, Lee SH, Kang HR, Suh DI. Successful desensitization of a patient with albumin hypersensitivity. Allergy Asthma Respir Dis. 2017; 5:117–20.

10. McLeod BC. Plasma and plasma derivatives in therapeutic plasmapheresis. Transfusion. 2012; 52(S1):38S–44S.

11. Hirayama F. Current understanding of allergic transfusion reactions: incidence, pathogenesis, laboratory tests, prevention and treatment. Br J Haematol. 2013; 160:434–44.

12. Matejtschuk P, Dash CH, Gascoigne EW. Production of human albumin solution: a continually developing colloid. Br J Anaesth. 2000; 85:887–95.

13. Heinonen J, Peltola K, Himberg JJ, Suomela H. Correlation of hypotensive effect of plasma protein fraction with prekallikrein activator activity: a clinical study in patients having open-heart surgery. Ann Thorac Surg. 1982; 33:244–9.

14. Alving BM, Hojima Y, Pisano JJ, Mason BL, Buckingham RE Jr, Mozen MM, et al. Hypotension associated with prekallikrein activator (Hage-man-factor fragments) in plasma protein fraction. N Engl J Med. 1978; 299:66–70.

15. Yu MW and Finlayson JS. Stabilization of human albumin by caprylate and acetyltryptophanate. Vox Sang. 1984; 47:28–40.
16. Hossaini AA, Hazeghi K, Amiri P. Experimental induction of caprylate-dependent albumin antibodies. Transfusion. 1977; 17:54–8.

17. Hafer C, Golla P, Gericke M, Eden G, Beutel G, Schmidt JJ, et al. Membrane versus centrifuge-based therapeutic plasma exchange: a randomized prospective crossover study. Int Urol Nephrol. 2016; 48:133–8.

18. Madore F. Plasmapheresis. Technical aspects and indications. Crit Care Clin. 2002; 18:375–92.
19. Myburgh JA and Mythen MG. Resuscitation fuids. N Engl J Med. 2013; 369:1243–51.
20. Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg. 2004; 139:552–63.

21. Mitra S and Khandelwal P. Are all colloids same? How to select the right colloid? Indian J Anaesth. 2009; 53:592–607.
22. Harm S, Schildböck C, Hartmann J. Removal of stabilizers from human serum albumin by adsorbents and dialysis used in blood purifcation. PLoS One. 2018; 13:e0191741.
Go to : 
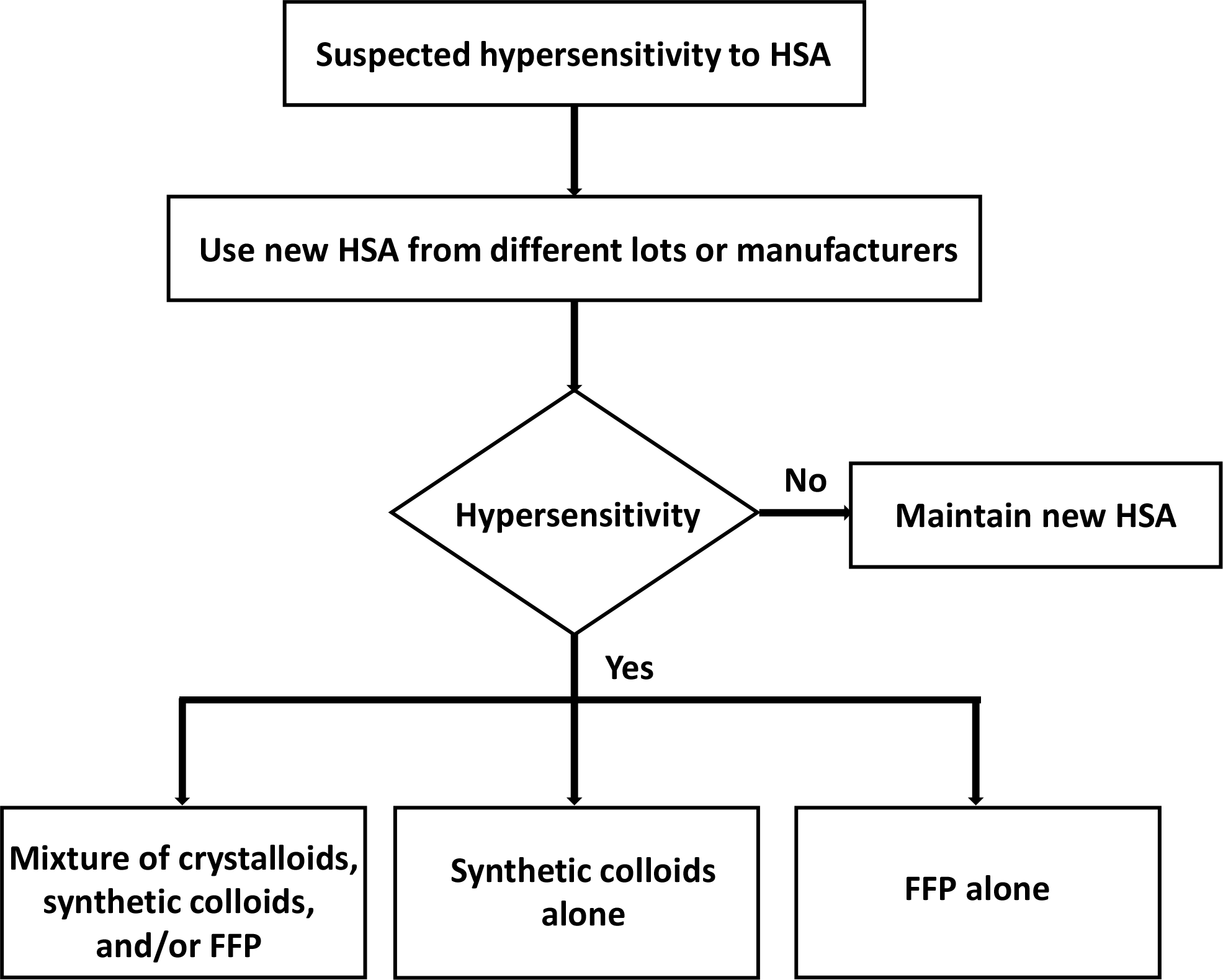 | Fig. 1.Proposed algorithm for replacement fluid selection when hypersensitivity to HSA is suspected during TPE. Replacement with HSA from different lots or manufacturers should be the first choice. If infusion of the new HSA still leads to hypersensitivity reactions, the following three options are recommended: 1) a mixture of crystalloids, synthetic colloids, and/or FFP, 2) synthetic colloids alone, or 3) FFP alone. Abbreviations: HSA, human serum albumin; FFP, fresh frozen plasma; TPE, therapeutic plasma exchange. |
Table 1.
Components of human serum albumin injection
| Components |
|---|
| Human albumin |
| Sodium chloride |
| Hydrochloric acid |
| Sodium hydroxide |
| Sodium N-acetyltryptophanate |
| Sodium caprylate |
| Water for injections |
Table 2.
Clinical features of the TPE procedures performed in the two patients




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download