This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
Implant wall thickness and the height of the implant-abutment interface are known as factors that affect the distribution of stress on the marginal bone around the implant. The goal of this study was to evaluate the long-term effects of supracrestal implant placement and implant wall thickness on maintenance of the marginal bone level.
Methods
In this retrospective study, 101 patients with a single implant were divided into the following 4 groups according to the thickness of the implant wall and the initial implant placement level immediately after surgery: 0.75 mm wall thickness, epicrestal position; 0.95 mm wall thickness, epicrestal position; 0.75 mm wall thickness, supracrestal position; 0.95 mm wall thickness, supracrestal position. The marginal bone level change was assessed 1 day after implant placement, immediately after functional loading, and 1 to 5 years after prosthesis delivery. To compare the marginal bone level change, repeated-measures analysis of variance was used to evaluate the statistical significance of differences within groups and between groups over time. Pearson correlation coefficients were also calculated to analyze the correlation between implant placement level and bone loss.
Results
Statistically significant differences in bone loss among the 4 groups (P<0.01) and within each group over time (P<0.01) were observed. There was no significant difference between the groups with a wall thickness of 0.75 mm and 0.95 mm. In a multiple comparison, the groups with a supracrestal placement level showed greater bone loss than the epicrestal placement groups. In addition, a significant correlation between implant placement level and marginal bone loss was observed.
Conclusions
The degree of bone resorption was significantly higher for implants with a supracrestal placement compared to those with an epicrestal placement.
Keywords: Dental implants, Dental implant-abutment design, Retrospective study
INTRODUCTION
Two approaches exist for explaining bone loss around implants. From a biological standpoint, reformation of the biological width, infection from microgaps, and peri-implantitis have been proposed as mechanisms underlying bone loss [
1]. From a mechanical standpoint, stress distribution around the crest module and body of the implant has been proposed as a factor causing bone loss, mostly based on finite element analysis (FEA) [
2345].
A previous FEA study suggested that the location of the implant-abutment interface and the implant wall thickness may be possible factors affecting the distribution of stress on the marginal bone around the implant. In that study, implants with a conical implant-abutment interface at the marginal bone level were compared to those with an implant-abutment interface located 2 mm more coronally. Additionally, various implant wall thicknesses (0.3 mm, 0.6 mm, or 0.9 mm) were examined. When the conical implant-abutment interface was at the level of the marginal bone, the high bone stresses caused by the axial load components were reduced by spatially separating them from those generated by the horizontal load components. However, when the interface was located more coronally, the positive effects of the conical interface disappeared. An additional analysis showed that increasing the wall thickness of the implant resulted in greater axial stiffness of the implant, thereby generating greater bone stress and loading on the uppermost side of the implant [
2]. Those patterns of behavior were also predicted by other FEA studies [
345], but have not been confirmed by clinical observations [
2].
In 2009, minor changes in the fixture design were made to Astra Tech implants (Astra Tech Dental Implant System, Astra Tech AB, Mölndal, Sweden). The newer fixtures have a greater wall thickness (0.95 mm compared with 0.75 mm), while maintaining a total fixture diameter of 4.0 mm. This change gave us the opportunity to investigate the effect of the wall thickness change on the marginal bone around the fixture.
Although it is recommended to place implants in the epicrestal position [
6], it sometimes is not possible to follow that recommendation, due to anatomical limitations, bone quality, and unexpected patient pain. Thus, the implant-abutment interface level could initially be located higher, without other factors that could influence bone level changes around implants. It was hypothesized that additional bone loss could occur because of bone stress arising from differences in the location of the implant-abutment interface and the wall thickness of implants. Thus, this study aimed to evaluate the effects of the wall thickness and depth of implants on bone loss through a retrospective study.
MATERIALS AND METHODS
Study setting and patients
This single-center, retrospective study was conducted using the records of 101 patients diagnosed with chronic periodontitis, who underwent implant surgery involving mucoperiosteal flap reflection on the premolar and molar areas at the Department of Periodontology of Gangnam Severance Dental Hospital between 2002 and 2011. The patients were followed up at 6-month intervals over a 5-year period. This study received approval from the Institutional Review Board of Yonsei University for retrospective chart review and data collection (3-2015-0170).
The patients were selected using the following inclusion criteria:
1) Absence of serious systemic conditions such as uncontrolled diabetes or hypertension.
2) Regular follow-up evaluations for at least 5 years.
3) If present, periodontitis was treated by surgical or non-surgical treatment, and maintenance therapy was performed at least twice a year during the follow up period.
4) Non-smoking status (or smoking fewer than 10 cigarettes per day).
5) Appropriate periapical radiographs for radiological assessments.
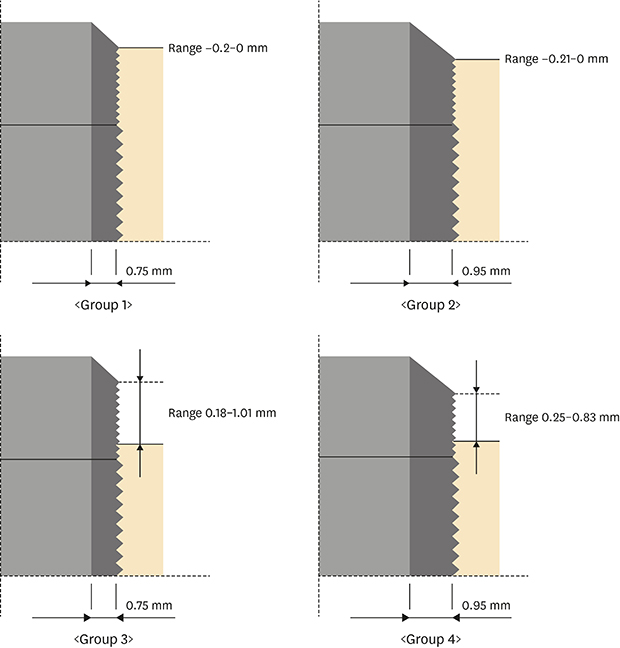
The patients were divided into 4 groups according to implant placement and wall thickness, with consideration of the male-to-female ratio and location of the implant (
Figure 1). Groups 1 and 2 had implants with a wall thickness of 0.75 mm and 0.95 mm, respectively, and all the implants were placed at or slightly below the marginal bone level (epicrestal placement) as per the manufacturer's guidelines (median distance from the crest: −0.10 mm and −0.12 mm, respectively). The reference point of the fixture was the border between the rough surface and the machined surface of the fixture. Groups 3 and 4 had implants with a wall thickness of 0.75 mm and 0.95 mm, respectively, with a supracrestal placement (median distance from the crest: 0.57 mm and 0.52 mm, respectively). There were 35, 37, 12, and 17 patients in groups 1–4, respectively.
 | Figure 1Initial implant placement level and wall thickness in the 4 groups. Range: initial maximum and minimum bone level of each group.
|
Implants
In this study, a total of 101 single internally hexed implants (Astra Tech Dental Implant System, Astra Tech AB) were used to replace missing premolars and molars. All implants had a microthreaded cervical neck, an internal hexagonal interface, a straight neck, and a diameter of 4.0 mm. The lengths of the implants varied from 8 mm to 11 mm. The distribution of the installed implants according to jaw and fixture is presented in
Table 1.
Table 1
The distribution of the installed implants according to jaw and placement site

|
Jaw |
Group |
Placement site |
Total |
|
7 |
6 |
5 |
4 |
4 |
5 |
6 |
7 |
|
Maxilla |
1 |
1 |
4 |
2 |
0 |
0 |
3 |
6 |
1 |
17 |
|
2 |
0 |
3 |
2 |
0 |
0 |
0 |
6 |
0 |
11 |
|
3 |
1 |
2 |
0 |
0 |
0 |
1 |
2 |
0 |
6 |
|
4 |
0 |
3 |
1 |
0 |
0 |
0 |
1 |
0 |
5 |
|
Mandible |
1 |
4 |
2 |
1 |
1 |
0 |
0 |
6 |
4 |
18 |
|
2 |
3 |
6 |
4 |
0 |
0 |
2 |
7 |
4 |
26 |
|
3 |
2 |
2 |
2 |
0 |
0 |
0 |
0 |
0 |
6 |
|
4 |
3 |
4 |
0 |
0 |
1 |
0 |
1 |
3 |
12 |
|
Total |
|
14 |
26 |
12 |
1 |
1 |
6 |
29 |
12 |
101 |

Surgical procedure
All surgical procedures were conducted by 2 periodontists who are board-certified in periodontology using a 2-stage treatment protocol. The second operation was performed 3 months and 6 months after the initial operation for the mandible and maxilla, respectively. Prostheses were delivered 3 weeks after the second operation. Patients were recalled every 6 months for plaque control and oral hygiene management.
Radiological examinations
Periapical radiographs (Eastman Kodak Co., Rochester, NY, USA) were taken 1 day after implant surgery, immediately after functional loading, 1 year after prosthesis delivery, and at annual follow-up visits with a commercially-available radiograph holder device (Extension cone paralleling kit, Rinn, Elgin, IL, USA) and a parallel cone technique (70 kV, 8 mA, 0.250 seconds) (
Figure 2). The X-ray tube was positioned at a 90° angle to the long axis of the dental implant in order to ensure that the implant threads were visible on both the mesial and distal images. To measure the length of the implants, a 5.5-mm spherical metal bearing was placed within the field of imaging. All films were developed using an automatic processor (Periomat, Dürr Dental, Bietigheim-Bissingen, Germany) according to the manufacturer's instructions. Digitization of the films was subsequently performed with an Epson GT-12000 (Epson, Nagano, Japan) at an output resolution of 2,400 dpi with 256 gray-scale.
 | Figure 2Periapical radiographs taken (A) 1 day after implant surgery, (B) immediately after functional loading, and at (C) 1 year, (D) 2 years, (E) 3 years, (F) 4 years, and (G) 5 years after prosthesis delivery.
|
Evaluation criteria and outcome measures
Measurements of marginal bone loss were made using ImageJ software (1.43e, National Institutes of Health, Bethesda, MD, USA). Calibrations were performed using the known diameter of a spherical metal bearing (5.5 mm). Changes in the peri-implant marginal bone level were then measured from the radiographs taken at baseline and the radiographs taken each year up to 5 years after prosthesis delivery. All radiographs were analyzed by the same examiner. One week later, radiographs from 30 randomly selected cases were remeasured to assess intra-examiner variability. In 93% of the measurements, the intra-examiner variability was <0.05 mm; in the remaining 7% of the measurements, the difference did not exceed 0.1 mm.
The margin between the polished surface and the rough surface of each implant was defined as the reference point. The distance between the reference point and the most apical point of the marginal bone was measured. Thus, the marginal bone level at the mesial and distal surfaces of the implant was assessed and averaged. The amount of marginal bone loss was measured yearly for up to 5 years. All measurements were compared among the 4 groups as outcome measures.
Statistical analysis
All calculations were performed on a personal computer using SPSS for Windows version 20.0 (IBM Corp., Armonk, NY, USA). The χ2 test was used to analyze the proportion of males and females and differences in implant locations. A repeated-measures analysis of variance (ANOVA) model was used with the bone loss level as the dependent variable and wall thickness and placement level as independent variables. Furthermore, the correlation between the implant placement level and bone loss was analyzed using Pearson correlation coefficients. The results of the statistical analysis were considered statistically significant if the P value was less than 0.05.
RESULTS
A total of 101 patients, comprising 41 men and 60 women, with a mean age of 56 years (range, 39–71 years) were analyzed in the present study. These patients were followed up at 6-month intervals over a 5-year period. There were no significant differences in the male-to-female ratio or the location of the implant among groups 1–4.
The results from the repeated-measures ANOVA analysis are presented in
Table 2. Significant differences were found between groups (
P<0.01) and within groups (
P<0.01). The
F-value of group was 16.386 (
P<0.01). The
F-value of time was 76.285 (
P<0.01).
Table 2
Repeated-measures ANOVA analysis result of bone loss for the 4 experimental groups over 5 years

|
Time |
Group |
F (P value) |
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group (A) |
Time (B) |
|
Year 1 |
0.04±0.07b)/b)
|
0.04±0.09b)/b)
|
0.18±0.13a)/d)
|
0.17±0.11a)/d)
|
13.415f) (0.000) |
|
|
Year 2 |
0.06±0.11b)/a)
|
0.07±0.14b)/a)
|
0.28±0.23a)/c)
|
0.28±0.20a)/c)
|
13.152f) (0.000) |
|
|
Year 3 |
0.07±0.13b)/a)
|
0.08±0.15b)/a)
|
0.38±0.34a)/b)
|
0.36±0.26a)/b)
|
15.658f) (0.000) |
|
|
Year 4 |
0.09±0.14b)/a)
|
0.09±0.17b)/a)
|
0.45±0.40a)/a)
|
0.44±0.31a)/a)
|
16.348f) (0.000) |
|
|
Year 5 |
0.09±0.13b)/a)
|
0.10±0.19b)/a)
|
0.49±0.44a)/a)
|
0.50±0.36a)/a)
|
17.408f) (0.000) |
|
|
F (P value) |
3.196e) (0.015) |
9.876f) (0.000) |
11.399f) (0.000) |
26.153f) (0.000) |
16.386f) (0.000) |
76.285f) (0.000) |
|
Comparison |
|
|
|
|
Group 3=Group 4>Group 1=Group 2 |
Year 5=Year 4>Year 3>Year 2>Year 1 |

At each time interval, there were significant bone loss changes except between group 1 and group 2 (epicrestal placement of the implant with a wall thickness of 0.75 mm and 0.95 mm, respectively) and between group 3 and group 4 (supracrestal placement of the implant with a wall thickness of 0.75 mm and 0.95 mm, respectively). In a multiple comparison, groups 3 and 4 showed more bone loss than groups 1 and 2. These results demonstrate that the initial implant placement level had a significant influence on further bone loss after 5 years of functioning, while wall thickness did not.
Bone loss over time was statistically significant in all 4 groups. The tendencies of bone loss over time in each group are shown in
Figure 3.
 | Figure 3 A tendency for bone resorption to increase over time was observed in each group based on mean values.
|
To examine whether a correlation existed between the implant-abutment interface level and marginal bone loss, 29 implants with a supracrestal placement level were examined. Because wall thickness had no significant effect for groups 3 and 4, those 2 groups were combined into a single group for this analysis. A statistically significant correlation between the depth of implant placement and marginal bone loss was observed (
Table 3).
Table 3
Pearson correlation coefficients between implant placement level and bone loss over time

|
Time |
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 |
|
Pearson correlation coefficient |
0.686a)
|
0.698a)
|
0.744a)
|
0.753a)
|
0.751a)
|
|
P value |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |

DISCUSSION
FEA is commonly applied to predict the pattern of stress distribution for implant components and the marginal bone involved [
278]. FEA models have helped researchers to simulate different clinical situations and to determine the best option from a biomechanical perspective. These aspects are of significant importance since stress distribution in peri-implant bone is a key factor affecting the success of dental implant placement [
9]. However, the findings of FEA studies on stress distribution are difficult to implement in clinical settings. FEA studies are also characterized by a lack of simulation of the inhomogeneous and anisotropic material properties of human bone. Additional analyses with detailed simulations of a more realistic bone model and simulations of clinical cases are still necessary, although analyses of material properties have clarified the effects of implant design on peri-implant bone stress. For these reasons, few clinical papers have investigated stress distribution in a way relevant to clinical situations [
10]. However, we had an opportunity by chance to conduct a clinical evaluation of factors analyzed in previous FEA studies. The clinical cases examined in the present study are similar to those used in the FEA study conducted by Hansson [
2], which studied the effects of implant wall thickness and the height of the abutment-implant interface on stress distribution.
Generally, practitioners aim to place implants in the epicrestal position during surgery. However, it is not always possible to achieve an ideal vertical position for implants, especially due to patient pain and surgical inexperience. As a result, variations occur in the depth of implant placement, and either subcrestal or supracrestal positioning of the interface can be achieved [
1112]. Based on these observations, we conducted the current study with the hypothesis that marginal bone loss might be affected by the location of the implant-abutment interface and implant wall thickness. The present study found that the degree of bone resorption was significantly higher for the implants with a supracrestal placement compared to those with an epicrestal placement. However, implant wall thickness did not affect marginal bone loss. The results of this study suggest the importance of the initial bone level in implant surgery. A supracrestal position of the implant-abutment interface may increase bone stress in the long term, thereby increasing the possibility of bone loss, which may cause peri-implantitis or implant failure. In contrast, the increased wall thickness of the implants seems to have had no significant influence on bone loss.
It is important to note that there is a discrepancy between the results of the previous FEA study [
2] and those of the present study regarding implant wall thickness, suggesting that the effects of implant wall thickness on stress loading may not be of clinical significance. The results of this study also confirmed that the depth of implant placement is a key factor associated with stress loading, rather than wall thickness.
A limitation of this study is that various factors can lead to bone loss [
1]. Although some mechanical factors, such as implant-abutment interface, implant shape, and the loading protocol were controlled, it was not possible to control for some other biological factors, such as peri-implantitis and individual susceptibility to periodontitis and peri-implantitis, which might influence bone loss. However, the patients were all enrolled in a periodontal maintenance program and strict plaque controls were performed at least twice a year, which might have minimized the biological effect of plaque on bone loss around the implant. Additionally, the initial implant level itself might have influenced the biological effect of plaque on the implant. For example, when implants were placed in the supracrestal position, the threads of each implant were exposed and plaque might have accumulated on them, potentially affecting the observed bone loss. Finally, the current study had a retrospective design and only a small series of patients was enrolled. In order to increase the number of subjects, we had to include some smokers (smoking fewer than 10 cigarettes/day), which might have influenced the observed patterns of bone loss. Therefore, large-scale, prospective, multi-center studies are still needed to confirm the present results. Within its limitations, the present study shows that the degree of bone resorption was significantly higher for implants with a supracrestal placement compared to those with an epicrestal placement. In addition, implant wall thickness did not affect marginal bone loss.







 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download