INTRODUCTION
MATERIALS AND METHODS
Animal preparation and definition of experimental groups
Surgical procedures
Micro-computed tomography analysis
Histological processing
Histomorphometric analysis
Statistical analysis
RESULTS
Micro–CT analysis
Table 1
Micro-computed tomography analysis

Histomorphometric analyses
Table 2
Histomorphometric analysis of the groups that underwent replantation after 5 minutes of dry storage

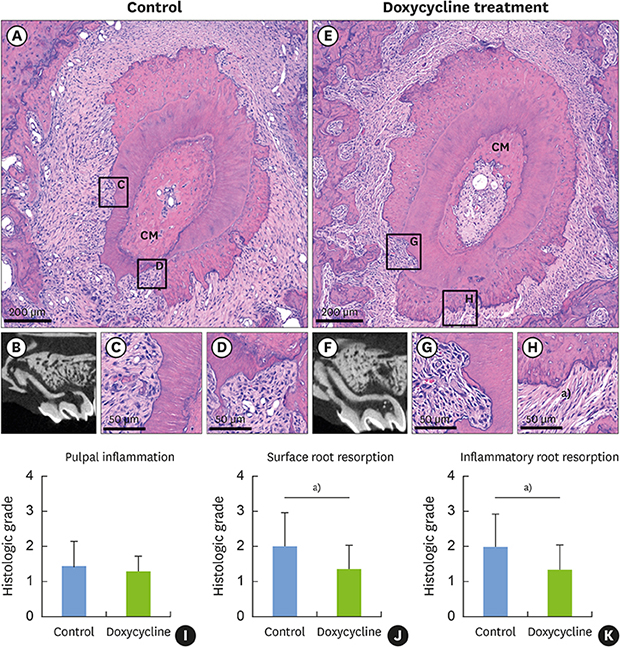
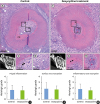 | Figure 2Sections of teeth replanted after 5 minutes of dry storage. (A) Gross histological characteristics of a representative root from group I reveals newly formed PDL-like tissues attached to most of the root surface, while some areas are without PDL-like tissue attachment, showing inflammatory resorption (asterisk). (B) Sagittal micro-CT image of the root in group I shows a visible CM in the canal space. (C) High magnification of the square region of the pulp in the control group shows disruption of the odontoblastic layer (arrows). (D) High magnification of the square region of the pulp proper in the control group shows inflammatory cells and fibroblasts in the pulp proper. (E) Gross histological characteristics of a representative root from group II reveals newly formed PDL-like tissues attached to most of the root surface, while some areas fail to exhibit PDL-like tissue attachment. (F) Sagittal micro-CT image of a root from group II. (G) High magnification of the square region of the pulp in the doxycycline group shows disruption of the odontoblastic layer (arrows). (H) High magnification of the square region of the pulp in the doxycycline group shows congestion of the blood vessels in the pulp proper. (I) Histologic grade of pulpal inflammation. (J) Histologic grade of surface root resorption. (K) Histologic grade of inflammatory resorption.PDL: periodontal ligament, CT: computed tomography, CM: calcified mass.
|
Table 3
Histomorphometric analysis in the groups that underwent replantation after 60 minutes of storage in HBSS

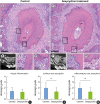 | Figure 3Sections of teeth replanted after 60 minutes of storage in HBSS. (A) Gross histological characteristics of a representative root from group III reveals root resorption in almost half of the root surface area and a CM densely embedded in the pulp canal. (B) Sagittal micro-CT image of the root from group II shows severe root resorption. (C) High magnification of the square region of the root in the control group shows a moderate degree of inflammatory resorption. (D) High magnification of the square region of the root from the control group shows severe inflammatory resorption extending beyond the cementum. (E) Gross histological characteristics of a representative root from group IV shows some degree of root resorption and a CM embedded in the pulp canal. (F) Sagittal micro-CT image of a root from Group IV. (G) High magnification of the square region of the root in the doxycycline group shows severe inflammatory resorption extending beyond the cementum. (H) High magnification of the square region of the root in the doxycycline group shows PDL-like tissue attachment in the presence of surface root resorption. (I) Histologic grade of pulpal inflammation. (J) A significantly lower histologic grade of surface root resorption was achieved in the doxycycline group (P=0.009). (K) A significantly lower histologic grade of inflammatory resorption was achieved in the doxycycline group (P=0.007).HBSS: Hank's balanced saline solution, CM: calcified mass, CT: computed tomography, PDL: periodontal ligament.
a)Statistically significant difference between the control and doxycycline groups (P<0.05, Mann-Whitney test).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download