CD14+ monocytes/macrophages in CPP crystal arthritis
In total, 19 patients with acute CPP crystal arthritis and 33 with acute gouty arthritis were included in this study.
Table 1 shows the clinical characteristics and laboratory findings for patients with acute CPP crystal arthritis and acute gouty arthritis. The mean age of the patients with acute CPP crystal arthritis was higher than that of patients with acute gouty arthritis (73.9±9.8 vs. 58.3±16.6; p<0.001). Patients with acute CPP crystal arthritis were predominantly women compared to those with acute gouty arthritis. However, there was no significant difference in leukocyte counts in the joint fluid between the 2 groups (
Table 1). Further, the difference among the frequencies of monocytes/macrophages in both group patients was examined. The population of monocytes/macrophages in SFMCs was identified by expression of CD14 but not of CD3, CD19, and CD56 (
Supplementary Fig. 1). The monocytes/macrophages were dominant among SFMCs, and their frequencies were not significantly different between acute CPP crystal and acute gout arthritis (
Fig. 1A). The frequency of lymphocytes identified by the expression of CD3, CD19, or CD56 was similar in the two groups. Next, when we determined the expression of markers which are characteristic of infiltrated monocytes (CD88, CCR2, MRP8, and MRP14) or tissue-resident macrophages (MERTK and 25F9) in CD14
+ monocytes/macrophages, these cells mainly expressed markers of infiltrated monocytes, such as CD88 (C5aR), CCR2, MRP8, and MRP14 (
Fig. 1B). The CCR2 expression was significantly higher, and CD88 expression was significantly lower in CD14
+ cells of acute CPP crystal arthritis than in those of acute gout. There was no significant expression of tissue-resident macrophages markers, such as MERTK and 25F9, in the 2 groups (
Fig. 1C). These data suggest that CD14
+ monocytes/macrophages in the synovial fluid of acute CPP crystal arthritis have the phenotype of infiltrating monocytes from the peripheral blood and that they comparatively showed different characteristics in their expression of migration-related receptors compared with those from patients with acute gouty arthritis.
Table 1
Clinical characteristics of patients with acute CPP crystal arthritis and acute gouty arthritis

|
Characteristics |
CPPD (n=19) |
Gout (n=33) |
p-value |
|
Age (yr) |
73.9±9.8 |
58.3±16.6 |
<0.001 |
|
Sex (male) |
6 (31.6) |
32 (97.0) |
<0.001 |
|
CRP [mg/dl, median (IQR)] |
12.2 (1.7) |
10.4 (1.5) |
0.434 |
|
Uric acid (mg/dl) |
4.6±2.2 |
7.2±2.5 |
<0.001 |
|
Joint fluid analysis |
|
|
|
|
WBC (/µl) |
24,246±15,503 |
39,641±49,763 |
0.197 |
|
Neutrophil [/µl, median (IQR)] |
21,263 (14,615) |
35,268 (42,006) |
0.168 |

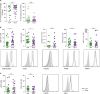 | Figure 1
Monocytes/macrophages population in synovial fluid of patients with acute CPP crystal and acute gouty arthritis. (A) The frequencies of monocytes/macrophages (left) and lymphocytes (right) in SFMCs of patients with acute CPP crystal arthritis (n=19) were compared with those of patients with gouty arthritis (n=33). (B, C) In the gating of CD14+CD3-CD19−CD56− cells, the marker expressions of infiltrated monocytes (B) and tissue-resident macrophages (C) was determined and compared between patients with acute CPP crystal arthritis (n=19) and acute gouty arthritis (n=26). Representative flow cytometry plots are presented.
*p<0.05.

|
Proinflammatory properties in monocytes/macrophages from synovial fluid of CPP crystal arthritis
To evaluate the functional characteristics of the CD14
+ cells during acute CPP crystal arthritis, we examined the expression of markers of proinflammatory (M1) and anti-inflammatory (M2) macrophages. There was no significant difference in the CD206 and CD80 expressions between the 2 groups (
Fig. 2A). However, CD14
+ cells in CPP crystal arthritis had significantly lower levels of CD163, one of the markers for alternatively activated macrophages (M2) than did the CD14
+ cells in acute gout. Then, we examined proinflammatory and anti-inflammatory cytokine production in the CD14
+ cells after stimulation with or without LPS (
Figs. 2B and C). There were no significant differences in the levels of proinflammatory cytokines (TNF-α and IL-1β) between the groups (
Fig. 2B). Conversely, IL-10 production was increased after LPS stimulation, but its levels were significantly lower in monocytes/macrophages from patients with acute CPP crystal arthritis (
Fig. 2C). These data suggest that synovial monocytes/macrophages in CPP crystal arthritis patients exhibit a more dominant proinflammatory phenotype than those in gout patients.
 | Figure 2
Pro- and anti-inflammatory phenotypes of CD14+ monocytes/macrophages in synovial cells. (A) CD206 (n=19 for CPP; n=30 for gout) and CD163 (n=19 for CPP; n=30 for gout) and CD80 (n=18 for CPP; n=19 for gout) were analysed from monocytes of patients with acute CPP crystal arthritis and acute gout. (B, C) Within the gate of CD14+CD3-CD19-CD56- cells, TNF-α and IL-1β (B) and IL-10 and IL-1Ra (C) were analysed after stimulation with/without LPS. The percentages of cytokine-producing cells from patients with acute CPP crystal arthritis (n=19) were compared with those from patients with acute gout (n=25).
IL-1Ra, IL-1 receptor antagonist.
*p<0.05; ***p<0.001.

|
CPP crystal-induced IL-8 may induce recruitment of neutrophils to inflamed synovium
Finally, the production of IL-6 and IL-8 (important factors for neutrophil migration) was examined (
Fig. 3). We found IL-8 expression in the CD14
+ cells from patients with acute CPP crystal arthritis. After stimulation with CPP crystals, the CD14
+ cells from acute CPP crystal arthritis patients produced more significant amounts of IL-8 but not of IL-6 (
Figs. 3A and B). To investigate the role of IL-8 in neutrophil infiltration during acute CPP crystal arthritis, the correlation between the frequency of IL-8-producing CD14
+ cells and the number of infiltrated neutrophils in the synovial fluid were analysed. We found a positive correlation between them, although statistical significance was not reached (
Fig. 3C). Collectively, these data suggest that IL-8 from infiltrated monocytes induces neutrophil migration into the joints of CPP crystal arthritis patients.
 | Figure 3
Correlation between the frequencies of neutrophils and IL-8-producing synovial monocytes. (A, B) In the gate of CD14+CD3−CD19−CD56− cells from patients with acute CPP crystal arthritis, the production of IL-8 and IL-6 was analysed following stimulation with or without CPP crystals. (A) Representative flow cytometry plots are presented. (B) The frequencies of IL-8- and IL-6-producing monocytes were compared before and after stimulation with CPP crystals. (C) The correlation between the infiltrated neutrophil number and IL-8+ monocytes/macrophages frequency in synovial fluid from patients with acute CPP crystal arthritis was analysed.
*p<0.05.

|
In this study, the frequency of CD14+ cells in acute CPP crystal arthritis was similar to that in acute gouty arthritis. This population of monocytes/macrophages showed the phenotype of infiltrating monocytes. The CD14+ cells from patients with acute CPP crystal arthritis had characterized by more pronounced inflammatory nature, as shown by the similar high levels of IL-1β and TNF-α production but significantly lower expression of IL-10 and CD163 compared with those from patients with acute gouty arthritis. The frequency of CD14+ cells expressing the neutrophil chemotactic factor IL-8 was correlated with neutrophil infiltration in the synovial fluid, and the IL-8 production was significantly induced by stimulation with CPP crystals.
Monocytes/macrophages are among the major responding cells in crystal-associated inflammation. MSU and CPP crystals stimulated the NLR family pyrin domain containing 3 inflammasome complex, leading to secretion of IL-1β and IL-18 from human monocytes/macrophages (
6). In a MSU crystal-induced inflammation model, peritoneal macrophages play an important role in initiating neutrophilic inflammation by producing IL-1β and TNF-α (
7). Previously, we reported that the frequency of CD14
+CD3
−CD19
−CD56
− cells was increased in the joints of patients with gout. These monocytes/macrophages exhibit proinflammatory phenotype and cause anti-inflammatory activity, such as IL-10 production and high expression of CD163, a marker of alternative activation of macrophages (M2) (
5). However, we observed that the CD14
+ cells from acute CPP crystal arthritis patients were more skewed towards proinflammatory as evidenced by similar significant increases in TNF-α and IL-1β but lower increases in IL-10 compared with those from acute gout patients (
Fig. 2). Further, CD163 expression was significantly lower in synovial fluid monocytes/macrophages of patients with acute CPP crystal arthritis. Thus, these findings lead to hypothesize that more pronounced inflammatory nature of CPP crystal arthritis contributes to the longer duration of symptoms of acute attack in pseudogout than in gout.
The recruitment of circulating monocytes into inflamed tissue plays an important role in the pathogenesis of a range of inflammatory disorders (
8). Our findings showed that CD14
+ cells had significant expression of CD88, CCR2, MRP8, and MRP14 but low expression of MERTK and 25F9. These results indicate that these cells during acute CPP crystal arthritis mainly exhibit phenotypes that resemble those of infiltrated monocytes rather than tissue-resident macrophages. However, chemokine receptors showed higher expression of CCR2 but lower expression of CD88 in acute CPP crystal arthritis than in gout (
Fig. 1B). Further studies are required to determine whether the mechanisms of monocyte migration during acute attack differ between pseudogout and gout.
A rapid and robust infiltration of neutrophils into the joint is a central feature during crystal-related arthropathy (
9). MSU and CPP crystals directly stimulate human monocytes to induce IL-6 or IL-8 which are key factors involved in neutrophil migration (
1011). In the present study, we directly examined IL-8 production from synovial fluid monocytes/macrophages
ex vivo during acute CPP crystal arthritis. A significant induction of IL-8 was detected after stimulation of synovial CD14
+ cells with CPP crystals in patients with acute CPP crystal arthritis (
Fig. 3B). Furthermore, we found that the frequency of IL-8 producing CD14
+ cells was correlated with the number of infiltrated neutrophils in synovial fluid (
Fig. 3C). However, the IL-6 production was not significantly induced after stimulation with CPP crystals. These findings suggest that IL-8, but not IL-6, derived from monocytes are responsible for neutrophilic inflammation in acute CPP crystal arthritis.
It will be interesting to determine whether the phenotypes of monocytes/macrophages are related to clinical characteristics, including resolution of acute CPP crystal arthritis. However, 14 (73.7%) of 19 patients with acute attacks received intra-articular corticosteroid injections to manage the symptoms. Thus, we could not address the differences in clinical characteristics according to differences in phenotypes of monocytes/macrophages with our study subjects because the majority of patients had dramatic improvement in their manifestations. Further studies addressing the relation between characteristics of monocytes/macrophages and clinical manifestations are warranted.
In conclusion, our study demonstrated that CD14+CD3−CD19−CD56− monocytes/macrophages in synovial fluid of patients with acute CPP crystal arthritis showed the phenotypes of infiltrated monocytes. These cells exhibited proinflammatory features, including production of TNF-α and IL-1β and expression of M1 marker. In addition, CD14+ cells had the capacity to induce IL-8 production in response to CPP crystals. Collectively, these results demonstrate that monocytes/macrophages in acute CPP crystal arthritis have a range of proinflammatory activity, including IL-8 secretion, possibly contributing to the robust and relatively sustained episodes of acute CPP crystal arthritis.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download